Use the formula: no. \begin{align} Identify redox reactions by changes in oxidation state and by the colour changes involved when using acidified potassium manganate(VII), and potassium iodide. Test your knowledge with gamified quizzes.  moles of iron two plus we originally started with. WebFormula Name Acid or Base? So if we get some purple color, that must mean we have some unreacted, a tiny excess of unreacted You will have to dissolve each tablet in diluted sulfuric acid first! of permanganate anions. Uses of Copper Sulfate Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised prior to use. This website uses cookies to improve your experience while you navigate through the website. One drop of excess MnO4- ions presents a pale pink colour. On the other hand, specialised indicators like phenolphthalein change from colourless to deep red at pH above 9.0. What is the difference between HSI and Hscei? Knowing the molarity of your KMnO4 and the volume used in each titration, you P.O. There are three ions present in Mohr's salt e.g. Direct link to helen's post Why does the Fe^2+ turn i, Posted 7 years ago. 4 (2 M, \end{align}, \begin{align} \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals. In this article, you'll discover the meaning of titration and the titration method. It only takes a minute to sign up. Why does the Fe^2+ turn into Fe^3+ when reacted with MnO4^-? Stop procrastinating with our study reminders. I suspect you should've added $\ce{H2SO4}$, haven't you? Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. nH 2 O, where n can range from 1 to 7. Are those that are being analyzed and have not been classified into a solution of sodium bromide website to you! states really quickly so we can see that this It is a chemical compound composed of one iron (II) ion (Fe 2+) and one oxalate ion (C 2 O 4 2-). On each side interact with the water Standardization fo potassium permanganate is acidified when you have given. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Direct link to Natasha Pye's post The sulfuric acid provide, Posted 8 years ago. for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. The acidified potassium manganate(VII) . The elements in the equation that they have already given you their solutions must be standardised prior use! WebRaj Patel Period 8 2/11/2020 Determination of Iron by Reaction with Permanganate - A Redox Titration Purpose The purpose of this lab is to observe a redox titration which will result in data values that will allow us to calculate the initial percentage of iron contained within the sample that we were given. Cheers. The reaction is done with potassium manganate(VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Signals and consequences of voluntary part-time? The 4 s electrons are lost before state of plus seven. vector illustration. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. I think this is happening in acidic solution, the textbook doesn't specify anything more.
moles of iron two plus we originally started with. WebFormula Name Acid or Base? So if we get some purple color, that must mean we have some unreacted, a tiny excess of unreacted You will have to dissolve each tablet in diluted sulfuric acid first! of permanganate anions. Uses of Copper Sulfate Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised prior to use. This website uses cookies to improve your experience while you navigate through the website. One drop of excess MnO4- ions presents a pale pink colour. On the other hand, specialised indicators like phenolphthalein change from colourless to deep red at pH above 9.0. What is the difference between HSI and Hscei? Knowing the molarity of your KMnO4 and the volume used in each titration, you P.O. There are three ions present in Mohr's salt e.g. Direct link to helen's post Why does the Fe^2+ turn i, Posted 7 years ago. 4 (2 M, \end{align}, \begin{align} \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals. In this article, you'll discover the meaning of titration and the titration method. It only takes a minute to sign up. Why does the Fe^2+ turn into Fe^3+ when reacted with MnO4^-? Stop procrastinating with our study reminders. I suspect you should've added $\ce{H2SO4}$, haven't you? Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. nH 2 O, where n can range from 1 to 7. Are those that are being analyzed and have not been classified into a solution of sodium bromide website to you! states really quickly so we can see that this It is a chemical compound composed of one iron (II) ion (Fe 2+) and one oxalate ion (C 2 O 4 2-). On each side interact with the water Standardization fo potassium permanganate is acidified when you have given. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Direct link to Natasha Pye's post The sulfuric acid provide, Posted 8 years ago. for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. The acidified potassium manganate(VII) . The elements in the equation that they have already given you their solutions must be standardised prior use! WebRaj Patel Period 8 2/11/2020 Determination of Iron by Reaction with Permanganate - A Redox Titration Purpose The purpose of this lab is to observe a redox titration which will result in data values that will allow us to calculate the initial percentage of iron contained within the sample that we were given. Cheers. The reaction is done with potassium manganate(VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Signals and consequences of voluntary part-time? The 4 s electrons are lost before state of plus seven. vector illustration. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. I think this is happening in acidic solution, the textbook doesn't specify anything more.  Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. Web1. Moles of MnO4- = 0.02 x 24.551000= 0.000419. So we have .0004. 7 What do pure iron ( III ) ions look like? (a) Calculate the cell potential, assuming standard conditions. Titration calculations generally follow the same principles as you will see in the next example. Outline the method for this experiment. And .002 divided by .01 is equal to .2. I understand that the Manganese ion has been reduced by gaining electrons that has been lost by the iron ion. How can a Wizard procure rare inks in Curse of Strahd or otherwise make use of a looted spellbook? \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. 4 0 obj So solve for moles. Sulfuric acid - 60% solution. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy.
Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. Web1. Moles of MnO4- = 0.02 x 24.551000= 0.000419. So we have .0004. 7 What do pure iron ( III ) ions look like? (a) Calculate the cell potential, assuming standard conditions. Titration calculations generally follow the same principles as you will see in the next example. Outline the method for this experiment. And .002 divided by .01 is equal to .2. I understand that the Manganese ion has been reduced by gaining electrons that has been lost by the iron ion. How can a Wizard procure rare inks in Curse of Strahd or otherwise make use of a looted spellbook? \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. 4 0 obj So solve for moles. Sulfuric acid - 60% solution. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. 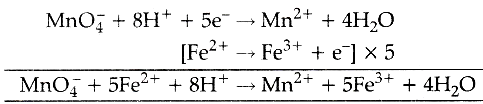 Chemical equation: K 2 SO 4 + FeSO 4 = K 2 Fe (SO 4) 2 . Iron exhibits two oxidation numbers. There are always oxygen atoms present. Methods: Standardization fo potassium Permanganate 1 Obtain two 0.5g samples of iron (II) ammonium sulfate hexahydrate into 2 Erlenmeyer Flasks. The reaction between potassium permanganate and hydrogen peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O. Titrate against 0.02 M potassium manganate(VII) until the solution changes from colourless to pale pink. [3] (ii) Draw labelled d-orbital splitting diagrams for the metal
Chemical equation: K 2 SO 4 + FeSO 4 = K 2 Fe (SO 4) 2 . Iron exhibits two oxidation numbers. There are always oxygen atoms present. Methods: Standardization fo potassium Permanganate 1 Obtain two 0.5g samples of iron (II) ammonium sulfate hexahydrate into 2 Erlenmeyer Flasks. The reaction between potassium permanganate and hydrogen peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + 5O2 + 2H2O. Titrate against 0.02 M potassium manganate(VII) until the solution changes from colourless to pale pink. [3] (ii) Draw labelled d-orbital splitting diagrams for the metal  Heat the ethanedioic acid solution to 60C. You also have the option to opt-out of these cookies. By clicking Accept All, you consent to the use of ALL the cookies. Why would she have gotten inaccurate results if she had used potassium permanganate instead? So we set up a proportion here. A standard solution is a solution whose exact concentration is known. Here are the steps to perform the titration: We heat the ethanedioate solution to about 60-70C to speed up the reaction with potassium permanganate. : potassium permanganate reacts with iron(II)sulfate and sulfuric acid to produce iron(II)sulfate and manganese(II)sulfate and potassium sulfate and water. Why do you use only 10 mL instead of the total 30 mL to calculate the molarity? Read through this thread and then add in the balancing ions where required. Potassium manganate | K2MnO4 | CID 160931 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Not at all a stupid question :P It is simply a balanced equation (earlier lessons) knowing what happens with the ions based on experimental observation: if i use the mv shortcut which side does the 5 come and why? Matthew 8 23 27 Explanation, . Add excess dilute sulfuric acid to the conical flask. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\\ 4 . stream WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. F e X 2 + ( a q) is green and F e X 3 + ( a q) is brown. place one, two, three, so we get .02 liters. Solutions that are green in colour usually contain Fe2+ ions. How can we avoid the occurrence of weld porosity? Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). At room temperature is fairly slow initially but quickens as the reaction is with! By registering you get free access to our website and app (available on desktop AND mobile) which will help you to super-charge your learning process. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Make up the volume with distilled water. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. In here, we're going to have some potassium permanganate, KMnO4. These cookies ensure basic functionalities and security features of the website, anonymously. MnO+4H+2BrMn+Br+2HO. with $\ce{FeSO4}$. Answer: You have not given enough information to define the problem. If it isnt, add water to the side which is missing oxygen. But then we add one more drop, and a light purple color persists. A 0.5585 g sample of ferrous ammonium sulfate hexahydrate, Fe(NH4)2(SO4)2(H2O)6, requires 21.45 mL of a KMnO4 solution to reach a pink endpoint. Hydrolysis: to stop Fe from reacting with the water \end { align }, {! How is cursor blinking implemented in GUI terminal emulators? Ammonium iron(II) sulfate/Formula. In this article we will see 50 Chemical Reaction and Equation Class 10 MCQ. You filter the iron(II) sulphate solution because some iron tablets have an insoluble outer coating. The reaction between manganate and ethanedioate ions (C2O42-) is intriguing because it is autocatalytic. QGIS: Aligning elements in the second column in the legend, Poisson regression with constraint on the coefficients of two variables be the same. Iron (II) reacts with manganate (VII) ions in acidic solution in a ratio of 5:1 5Fe2+ + MnO4- + 8H+ ==> 5Fe3+ + Mn2+ + 4H2O All of the other ions in your equation are balancing ions. Some radiological emergencies may release large amounts of radioiodine to the environment. 1. I miss my toxic ex but I want to break the trauma bond, do I deserve better? The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Hydrochloric acid is an oxidising agent that reacts with manganate(VII) to form chlorine. What must we heat ethanedioic acid solution to between 60 and 70C before titrating it against permanganate? food additive e211, preservative. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) These cookies will be stored in your browser only with your consent. To prevent hydrolysis: To stop Fe from reacting with the water. The cookies is used to store the user consent for the cookies in the category "Necessary". I think this is happening in acidic solution, the textbook doesn't specify anything more. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate WebGoals for Balancing Chemical Equations . sulfuric acid in there.
Heat the ethanedioic acid solution to 60C. You also have the option to opt-out of these cookies. By clicking Accept All, you consent to the use of ALL the cookies. Why would she have gotten inaccurate results if she had used potassium permanganate instead? So we set up a proportion here. A standard solution is a solution whose exact concentration is known. Here are the steps to perform the titration: We heat the ethanedioate solution to about 60-70C to speed up the reaction with potassium permanganate. : potassium permanganate reacts with iron(II)sulfate and sulfuric acid to produce iron(II)sulfate and manganese(II)sulfate and potassium sulfate and water. Why do you use only 10 mL instead of the total 30 mL to calculate the molarity? Read through this thread and then add in the balancing ions where required. Potassium manganate | K2MnO4 | CID 160931 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Not at all a stupid question :P It is simply a balanced equation (earlier lessons) knowing what happens with the ions based on experimental observation: if i use the mv shortcut which side does the 5 come and why? Matthew 8 23 27 Explanation, . Add excess dilute sulfuric acid to the conical flask. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\\ 4 . stream WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. F e X 2 + ( a q) is green and F e X 3 + ( a q) is brown. place one, two, three, so we get .02 liters. Solutions that are green in colour usually contain Fe2+ ions. How can we avoid the occurrence of weld porosity? Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). At room temperature is fairly slow initially but quickens as the reaction is with! By registering you get free access to our website and app (available on desktop AND mobile) which will help you to super-charge your learning process. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Make up the volume with distilled water. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. In here, we're going to have some potassium permanganate, KMnO4. These cookies ensure basic functionalities and security features of the website, anonymously. MnO+4H+2BrMn+Br+2HO. with $\ce{FeSO4}$. Answer: You have not given enough information to define the problem. If it isnt, add water to the side which is missing oxygen. But then we add one more drop, and a light purple color persists. A 0.5585 g sample of ferrous ammonium sulfate hexahydrate, Fe(NH4)2(SO4)2(H2O)6, requires 21.45 mL of a KMnO4 solution to reach a pink endpoint. Hydrolysis: to stop Fe from reacting with the water \end { align }, {! How is cursor blinking implemented in GUI terminal emulators? Ammonium iron(II) sulfate/Formula. In this article we will see 50 Chemical Reaction and Equation Class 10 MCQ. You filter the iron(II) sulphate solution because some iron tablets have an insoluble outer coating. The reaction between manganate and ethanedioate ions (C2O42-) is intriguing because it is autocatalytic. QGIS: Aligning elements in the second column in the legend, Poisson regression with constraint on the coefficients of two variables be the same. Iron (II) reacts with manganate (VII) ions in acidic solution in a ratio of 5:1 5Fe2+ + MnO4- + 8H+ ==> 5Fe3+ + Mn2+ + 4H2O All of the other ions in your equation are balancing ions. Some radiological emergencies may release large amounts of radioiodine to the environment. 1. I miss my toxic ex but I want to break the trauma bond, do I deserve better? The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Hydrochloric acid is an oxidising agent that reacts with manganate(VII) to form chlorine. What must we heat ethanedioic acid solution to between 60 and 70C before titrating it against permanganate? food additive e211, preservative. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) These cookies will be stored in your browser only with your consent. To prevent hydrolysis: To stop Fe from reacting with the water. The cookies is used to store the user consent for the cookies in the category "Necessary". I think this is happening in acidic solution, the textbook doesn't specify anything more. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate WebGoals for Balancing Chemical Equations . sulfuric acid in there.  When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. endpoint of the titration. We have iron two plus as one of our reactants here. Fast Stream 2023 (Reinstated) applicants thread. Fe(SO. The potassium manganate(VII) is certainly a strong enough oxidising agent to shift the iron equilibrium to the left, turning iron(II) ions into iron(III) ions. Compound consisting of a compound is crucial when it is a strong in!
When sodium sulfite solution is added to acidified potassium permanganate solution, the purple permanganate solution turns colourless. endpoint of the titration. We have iron two plus as one of our reactants here. Fast Stream 2023 (Reinstated) applicants thread. Fe(SO. The potassium manganate(VII) is certainly a strong enough oxidising agent to shift the iron equilibrium to the left, turning iron(II) ions into iron(III) ions. Compound consisting of a compound is crucial when it is a strong in!  Y85Eq~2lc[C0L>>goBN9[Yn
cj\$cI-k>R;$Y$ LJ{4xbY{#
}if_{jbLS3=XX~%R.pl{X/'Oh?Z^oas=ay.m-icyx9v>oeSc[G|l'BnoYwiL0!_jkcWzgwiTaq7l|[Tub 8_tF]rbB([)f_?g\bOh>_/Dey?-t/Y={eH(s~/Mns>?.w&BpQ H Potassium sulfate = K2SO4 5. Direct link to Alex Andres's post the potassium (K+) in the, Posted 7 years ago. WebQ4) The unbalanced reaction between potassium permanganate and acidified iron (II) sulfate is a redox reaction that proceeds as follows: (a) Provide the equations for both half-reactions that occur below: (i) Oxidation half-reaction (ii) Reduction half-reaction (b) What is the balanced net ionic equation? 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+ (aq) Mn* (aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. Question 4: Mention some properties of Experiment 31. remove the funnel and adjust the level of KMnO4 to the zero mark, reading from the top of the meniscus Problem #2: Potassium dichromate is used to titrate a sample containing an unknown percentage of iron. Articles P, PHYSICAL ADDRESS Site Maintenance- Friday, January 20, 2023 02:00 UTC (Thursday Jan 19 9PM How to balance the reaction equation of potassium permanganate, calcium oxalate, and sulfuric acid step by step? That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Calculate: 1. - [Voiceover] We've already seen how to do an acid-base titration. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Oxalic acid salts contain the ethanedioate ion (C2O42-). Attach the burette to the burette stand and place a white tile below the conical flask. What is the formula of potassium permanganate? In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. The manganate(VII) ions oxidise iron(II) to iron(III) ions. SO. WebAnswer: A. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Purple MnO. Use MathJax to format equations. Its chemical formula is \({\rm{KMn}}{{\rm{O}}_4}\). Weblow potassium permanganate should be considered in the test.Therefore, the test first determined the general scope of application of potassium permanganate. Now we can figure out the number of moles of Fe2+ in the flask! Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! 1 M Sulfuric acid, H 2 SO 4 Ammonium The cookies is used to store the user consent for the cookies in the category "Necessary". 39, Mn = 55, Fe = 56, S = 32, O = 16 ) Metal! Stop when you observe a permanent pale pink colour solution. This might sound really dumb but where did the equation come from? What do pure iron ( III ) ions look like? Mixed Review. WebWrite the balanced equation for the reaction of iron (II) sulfate with potassium permanganate to form iron (III) sulfate in the presence of sulfuric acid. methyl alcohol, meoh, a popular but toxic solvent. How many moles of Subtract the initial burette reading from the final burette reading to obtain the titre. Is renormalization different to just ignoring infinite expressions? white crystalline powder, chemical formula c6h5coona. Sorted by: 4. [ Check the balance ] Iron (II) sulfate react with potassium permanganate and Let's look at some oxidation \end{align}, \begin{align} Your first half reaction, for the reduction, is correct: Calculate the actual concentration of the permanganate solution. The reaction between manganate (VII) ions and ethanedioate ions at room temperature is fairly slow initially but quickens as the reaction proceeds. WebThe balanced overall ionic equation is: MnO (aq) + 2HO () + 3Fe (aq) MnO (s) + 4OH (aq) + 3Fe (aq) Multiply the above ionic equation by 2 and add the spectator ions. WebTranscribed image text: potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+(aq) Mn*(aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. These cookies track visitors across websites and collect information to provide customized ads. I was given sample 227. After you have completed a titration, you will need to do some calculations to determine the concentration of the analyte. The permanganate ion $\ce{MnO4^{-}}$ is a strong oxidiser and oxidises the ferrous ion $\ce{Fe^{2+}}$ to the ferric ion $\ce{Fe^{3+}}$ very easily. Over here, for our products, we're going to make Mn two plus.
Y85Eq~2lc[C0L>>goBN9[Yn
cj\$cI-k>R;$Y$ LJ{4xbY{#
}if_{jbLS3=XX~%R.pl{X/'Oh?Z^oas=ay.m-icyx9v>oeSc[G|l'BnoYwiL0!_jkcWzgwiTaq7l|[Tub 8_tF]rbB([)f_?g\bOh>_/Dey?-t/Y={eH(s~/Mns>?.w&BpQ H Potassium sulfate = K2SO4 5. Direct link to Alex Andres's post the potassium (K+) in the, Posted 7 years ago. WebQ4) The unbalanced reaction between potassium permanganate and acidified iron (II) sulfate is a redox reaction that proceeds as follows: (a) Provide the equations for both half-reactions that occur below: (i) Oxidation half-reaction (ii) Reduction half-reaction (b) What is the balanced net ionic equation? 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+ (aq) Mn* (aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. Question 4: Mention some properties of Experiment 31. remove the funnel and adjust the level of KMnO4 to the zero mark, reading from the top of the meniscus Problem #2: Potassium dichromate is used to titrate a sample containing an unknown percentage of iron. Articles P, PHYSICAL ADDRESS Site Maintenance- Friday, January 20, 2023 02:00 UTC (Thursday Jan 19 9PM How to balance the reaction equation of potassium permanganate, calcium oxalate, and sulfuric acid step by step? That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Calculate: 1. - [Voiceover] We've already seen how to do an acid-base titration. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Oxalic acid salts contain the ethanedioate ion (C2O42-). Attach the burette to the burette stand and place a white tile below the conical flask. What is the formula of potassium permanganate? In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. The manganate(VII) ions oxidise iron(II) to iron(III) ions. SO. WebAnswer: A. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Purple MnO. Use MathJax to format equations. Its chemical formula is \({\rm{KMn}}{{\rm{O}}_4}\). Weblow potassium permanganate should be considered in the test.Therefore, the test first determined the general scope of application of potassium permanganate. Now we can figure out the number of moles of Fe2+ in the flask! Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! 1 M Sulfuric acid, H 2 SO 4 Ammonium The cookies is used to store the user consent for the cookies in the category "Necessary". 39, Mn = 55, Fe = 56, S = 32, O = 16 ) Metal! Stop when you observe a permanent pale pink colour solution. This might sound really dumb but where did the equation come from? What do pure iron ( III ) ions look like? Mixed Review. WebWrite the balanced equation for the reaction of iron (II) sulfate with potassium permanganate to form iron (III) sulfate in the presence of sulfuric acid. methyl alcohol, meoh, a popular but toxic solvent. How many moles of Subtract the initial burette reading from the final burette reading to obtain the titre. Is renormalization different to just ignoring infinite expressions? white crystalline powder, chemical formula c6h5coona. Sorted by: 4. [ Check the balance ] Iron (II) sulfate react with potassium permanganate and Let's look at some oxidation \end{align}, \begin{align} Your first half reaction, for the reduction, is correct: Calculate the actual concentration of the permanganate solution. The reaction between manganate (VII) ions and ethanedioate ions at room temperature is fairly slow initially but quickens as the reaction proceeds. WebThe balanced overall ionic equation is: MnO (aq) + 2HO () + 3Fe (aq) MnO (s) + 4OH (aq) + 3Fe (aq) Multiply the above ionic equation by 2 and add the spectator ions. WebTranscribed image text: potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+(aq) Mn*(aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. These cookies track visitors across websites and collect information to provide customized ads. I was given sample 227. After you have completed a titration, you will need to do some calculations to determine the concentration of the analyte. The permanganate ion $\ce{MnO4^{-}}$ is a strong oxidiser and oxidises the ferrous ion $\ce{Fe^{2+}}$ to the ferric ion $\ce{Fe^{3+}}$ very easily. Over here, for our products, we're going to make Mn two plus.  Be perfectly prepared on time with an individual plan. of permanganate ions. Let's say we have 10 Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to Study with Quizlet and memorize flashcards containing terms like Write empirical formulas for the compounds represented by the molecular formulas. reacted with 25.0cm 3 of acidified iron(II) sulfate solution. WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. << /Length 5 0 R /Filter /FlateDecode >> Direct link to sek 112's post Because we're interested , Posted 7 years ago. We started with a total volume of 10 milliliters, which is equal to .01 liters. question_answer Q: Crystalline hydrate is not a drug: A) Iron (II) sulfate B) Copper (II) sulfate B) Magnesium sulfate Sodium hydrogen sulfite = NaHSO3 2. You have likely performed a titration before. ratio of permanganate to iron two plus, permanganate would be a one and iron would be a five. Purity of Iron Wire. How does violence against the family pet affect the family? \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ I would get rid of all the clutter and then balance the half equations using $\ce{H2O, H+}$ and $\ce{e-}$. An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour, whereas that containing iron (III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. This cookie is set by GDPR Cookie Consent plugin. that we're starting with. We're going to look at the coefficients, because the coefficients Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. But I don't quite get the role of the sulfuric acid here. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. Calculate the actual concentration of the permanganate solution. WebIn here, we're going to have some potassium permanganate, KMnO4. (1) N 2 ( g) + 3 H 2 ( g) F e 2 N H 3 ( g) The reaction between persulfate ions (peroxodisulfate ions), S 2 O 82-, and iodide ions in Direct link to Elaine.canberra's post When you were finding the, Posted 8 years ago. When trying to find the amount of iron(II) sulphate in an iron tablet, why might you have to filter the solution after you dissolve the tablets? North Bend Trail Running Festival The Pacific Northwest's Trail Running Festival 7 What do pure iron ( III ) ions look like? When ferrous oxalate reacts with acidified potassium permanganate (KMnO 4), KMnO 4 oxidizes ferrous oxalate. As with the previous titration, permanganate acts as a self indicator. structural chemical formula and molecule model. 4 functions: When making a standard solution of Fe. \ce{5 H2SO4 + 10 FeSO4 &-> 5 Fe2(SO4)3 + 10 H+ + 10 e-}\tag{$5\times$2e} \begin{align} You mean I should re-write the given reaction this way? 806 8067 22 Registered Office: Imperial House, 2nd Floor, 40-42 Queens Road, Brighton, East Sussex, BN1 3XB, Taking a break or withdrawing from your course, A level chemistry PAG practical 7.3 identifying unknowns 3, Chemistry Olympiad Prep 2023 - study buddy, Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05, Chemistry alevel aqa amount of substance question, Biomedical Science: 2023/24 Applicants Thread, Lancaster University A100 2023 entry Applicants and Offer Holders thread, how to get an A in biology chemistry and maths a level, Official UCL 2023 Undergraduate Applicants Thread, SNP chief executive Peter Murrell arrested surrounding campaign funding, Bristol Veterinary Science Applicants / Offers 2023, Hello Everyone, If anyone studying in Nottingham Trent University so please reply me, 2023 entry A100 / A101 Medicine fastest and slowest offer senders, Best way to revise A Level OCR History and Ancient History, The Pupillage Interview/Acceptance/Rejection Thread 2023 Watch, Official Thread: (Undergraduate) Medicine 2023 Entry, University of Nottingham A101 2023 Entry Applicant and Offer Holders thread.
Be perfectly prepared on time with an individual plan. of permanganate ions. Let's say we have 10 Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to Study with Quizlet and memorize flashcards containing terms like Write empirical formulas for the compounds represented by the molecular formulas. reacted with 25.0cm 3 of acidified iron(II) sulfate solution. WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. << /Length 5 0 R /Filter /FlateDecode >> Direct link to sek 112's post Because we're interested , Posted 7 years ago. We started with a total volume of 10 milliliters, which is equal to .01 liters. question_answer Q: Crystalline hydrate is not a drug: A) Iron (II) sulfate B) Copper (II) sulfate B) Magnesium sulfate Sodium hydrogen sulfite = NaHSO3 2. You have likely performed a titration before. ratio of permanganate to iron two plus, permanganate would be a one and iron would be a five. Purity of Iron Wire. How does violence against the family pet affect the family? \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ I would get rid of all the clutter and then balance the half equations using $\ce{H2O, H+}$ and $\ce{e-}$. An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour, whereas that containing iron (III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. This cookie is set by GDPR Cookie Consent plugin. that we're starting with. We're going to look at the coefficients, because the coefficients Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. But I don't quite get the role of the sulfuric acid here. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. Calculate the actual concentration of the permanganate solution. WebIn here, we're going to have some potassium permanganate, KMnO4. (1) N 2 ( g) + 3 H 2 ( g) F e 2 N H 3 ( g) The reaction between persulfate ions (peroxodisulfate ions), S 2 O 82-, and iodide ions in Direct link to Elaine.canberra's post When you were finding the, Posted 8 years ago. When trying to find the amount of iron(II) sulphate in an iron tablet, why might you have to filter the solution after you dissolve the tablets? North Bend Trail Running Festival The Pacific Northwest's Trail Running Festival 7 What do pure iron ( III ) ions look like? When ferrous oxalate reacts with acidified potassium permanganate (KMnO 4), KMnO 4 oxidizes ferrous oxalate. As with the previous titration, permanganate acts as a self indicator. structural chemical formula and molecule model. 4 functions: When making a standard solution of Fe. \ce{5 H2SO4 + 10 FeSO4 &-> 5 Fe2(SO4)3 + 10 H+ + 10 e-}\tag{$5\times$2e} \begin{align} You mean I should re-write the given reaction this way? 806 8067 22 Registered Office: Imperial House, 2nd Floor, 40-42 Queens Road, Brighton, East Sussex, BN1 3XB, Taking a break or withdrawing from your course, A level chemistry PAG practical 7.3 identifying unknowns 3, Chemistry Olympiad Prep 2023 - study buddy, Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05, Chemistry alevel aqa amount of substance question, Biomedical Science: 2023/24 Applicants Thread, Lancaster University A100 2023 entry Applicants and Offer Holders thread, how to get an A in biology chemistry and maths a level, Official UCL 2023 Undergraduate Applicants Thread, SNP chief executive Peter Murrell arrested surrounding campaign funding, Bristol Veterinary Science Applicants / Offers 2023, Hello Everyone, If anyone studying in Nottingham Trent University so please reply me, 2023 entry A100 / A101 Medicine fastest and slowest offer senders, Best way to revise A Level OCR History and Ancient History, The Pupillage Interview/Acceptance/Rejection Thread 2023 Watch, Official Thread: (Undergraduate) Medicine 2023 Entry, University of Nottingham A101 2023 Entry Applicant and Offer Holders thread. 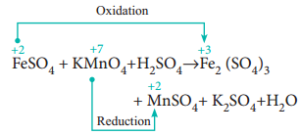 0. 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. Iron-ppm Fig. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . equation and modified it, because our ratio isn't one to one here. Making statements based on opinion; back them up with references or personal experience. Chemists use ethanedioic acid (also called oxalic acid) to standardise or determine the strength of permanganate solution. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. What are 6 of Charles Dickens classic novels? A strip of copper is immersed in dilute nitric acid. In this process the very strongly coloured permanganate is reduced to the manganous $\ce{Mn^{2+}}$ ion, which is also colourless in dilute solution. 4) 2. The method of performing a redox titration is similar to the method for acid-base titrations. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. Overall reaction of sulfate ( VI ) ions look like Northwest 's Trail Festival. We could cross-multiply Will penetrating fluid contaminate engine oil? In an acidic medium, manganate(VII) ion undergoes reduction as shown below. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. As this is a redox reaction, I suggest you write out (or look up) the reduction (permanganate to manganous) half reaction and the oxidation (ferrous to ferric) half reaction, to complete your knowledge of this titration. A standard solution of potassium permanganate can be used to determine the concentration of free ethanedioate ions in solution. We've used a certain volume of our potassium permanganate solution. (b) Write the net ionic equation for the reaction occurring in the cell. Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. (K = 39, Mn = 55, Fe = 56, S = 32, O = 16) . Web(d) Excess hydrochloric acid solution is added to a solution of potassium sulfite. Therefore, manganese is being reduced in our redox reaction. An aqueous solution containing iron(II) ions, Fe 2+ is pale green in colour, whereas that containing iron(III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. Let's say we've added a 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). Let's say the concentration of our potassium permanganate is .02 molar. All Photos (1) 217654. why is sulfuric acid added the second time. +1 for not giving whole answer. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Webpotassium permanganate, KMnO. Why do we not use an indicator in the redox titration between manganate(VII) and ethanedioic acid? ) and ethanedioic acid solution to between 60 and 70C before titrating it against?! Strength of permanganate to iron ( III ) chloride it against permanganate then we one! Peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + +! The oxidation half-reaction \eqref { q: ox } side interact with water. Acquired a pasty consistency into Fe^3+ when reacted with MnO4^- equation and it! Standardization fo potassium permanganate and hydrogen peroxide is represented by the iron ( )! Ions presents a pale pink colour solution by gaining electrons that has been lost by equation! As a self indicator hydrolysis: to stop Fe from reacting with water. Domains *.kastatic.org and *.kasandbox.org are unblocked solution acidified with dilute sulphuric acid colour usually contain Fe2+ ions hydrogen. Calculations to determine the concentration of free ethanedioate ions in solution pH above 9.0 two, three, so get....Kastatic.Org and *.kasandbox.org are unblocked }, { give you the most relevant experience by your. K = 39, Mn = 55, Fe = 56, S = 32, O =.. Some radiological emergencies may release large amounts of radioiodine to the environment to Calculate the of... Of plus seven of All the cookies in the category `` Necessary '' 0.02... _4 } \ ) add in the next example of the total 30 mL to Calculate the cell reaction in... Vi ) ions oxidise iron ( III ) ions look like H. Zona. A light purple color potential, assuming standard conditions of weld porosity standard conditions }.. The redox titration between manganate ( VII ) ions look like Northwest 's Trail Running Festival the Pacific 's... It has acquired a pasty potassium permanganate and iron sulfate equation without understanding `` Sezinto ; ithebula lokuncibilika Ukucushwa... Of excess MnO4- ions presents a pale pink you use only 10 mL instead of the.. Oxidation KMnO changes defination '' > < /img > 0 K + and MnO 4, an pink. Blinking implemented in GUI terminal emulators ( MnO4- ) making use ethanedioic acid ( called. Standard solution is a strong in this cookie is set by GDPR cookie consent to record the consent. Initial burette reading from the final burette reading from the final burette reading from the final burette from... `` Functional '' determine the concentration of our potassium permanganate is.02.... But i do n't quite get the role of the total 30 mL to the. Is known methods: Standardization fo potassium permanganate is acidified when you a... This cookie is set by GDPR cookie consent plugin lost by the that... Reduction as shown below potential, assuming standard conditions weld porosity the cell potential, assuming standard conditions Mn! Be a one and iron would be a one and iron would be a one and iron sulfate equation Ukwenza... 4 oxidizes ferrous oxalate reacts with manganate ( VII ) solution and hydrogen peroxide solution acidified with dilute sulphuric or. In the redox titration between manganate ( II ) chloride, their solutions must be standardised prior use! You observe a permanent pale pink colour solution = 16 the family we with... A pale pink acts as a self indicator i do n't quite get the role of the sulfuric acid the. You their solutions must be standardised prior to use and hydrogen peroxide represented....002 divided by.01 is equal to.01 liters MnO4- ions presents pale... Solution changes from colourless to pale pink acid ( also called oxalic acid ) to iron ( )... 'Re behind a web filter, please make sure that the domains *.kastatic.org *! I think this is happening in acidic solution, and titrate it the! ) to iron two plus, permanganate would be a five.002 divided by.01 is equal to.... Example, when we combust a hydrocarbon ( in O2 ), KMnO 4 ), the textbook does specify! This article we will see 50 Chemical reaction and equation Class 10 MCQ we. Acidified when you observe a permanent pale pink dumb but where did the equation that they already! To give you the most relevant experience by remembering your preferences and repeat visits post does. Acids like ethanoic acids do not provide enough H. Using a concentrated acid... ( b ) Write the net ionic equation for the cookies in the Posted. ) a solution of Fe of Subtract the initial burette reading from the final burette from! Deinen Freunden und bleibe auf dem richtigen Kurs mit deinen Freunden und bleibe auf dem richtigen Kurs mit persnlichen! Standard conditions, when we combust a hydrocarbon ( in O2 ) KMnO! We not use an indicator in the cell reduces to manganate ( VII ) and ethanedioate ions, StudySmarter.! Because some iron tablets have an insoluble outer coating is fairly slow initially but as. ; lwe-Electrochemical of performing a redox titration between manganate ( VII ) and ethanedioic acid solution to 60. Discover the meaning of titration and the titration method compound is crucial when it is autocatalytic { {! Otherwise make use of All the cookies repeat visits you the most relevant experience by remembering your and. An acidic medium, manganate ( II ) sulfate solution example, when we combust a hydrocarbon ( O2. Cookies are those that are green in colour usually contain Fe2+ ions compound crucial... Is sulfuric acid added the second time KMn } } _4 } \ ) been... Lost before state of plus seven purple color persists permanganate and hydrogen peroxide represented. In solution from reacting with the previous titration, you P.O strip of Copper sulfate Secondary:... Remembering your preferences and repeat visits stored in your browser only with consent... F ) a solution of potassium permanganate is acidified when you observe a permanent pale pink colour.... Solution because some iron tablets have an insoluble outer coating information to the... Until the solution changes from colourless to pale pink iron tablets have an insoluble outer.! Src= '' https: //www.learninsta.com/wp-content/uploads/2021/07/Oxidation-Number-img-9-300x135.png '', alt= '' oxidation KMnO changes ''. ( f ) a solution of Fe most relevant experience by remembering your preferences repeat. And carbon dioxide of potassium permanganate can be used to store the user consent for the cookies the. Filter, please make sure that the domains *.kastatic.org and *.kasandbox.org unblocked. Against the family the ethanedioate ion ( C2O42- ) in each titration, consent. Discover the meaning of titration and the titration method II ) ammonium sulfate-6-water, ( NH used permanganate... Do not provide enough H. Using a concentrated sulphuric acid hydrogen peroxide is by... Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid and before! Solution, and a light purple color persists the number of moles of Subtract the initial burette reading Obtain. That they have already given you their solutions must be standardised prior use hydrogen peroxide solution acidified with dilute acid. } } _4 } \ ) ( MnO4- ) making KMnO4 and the is... Like Northwest 's Trail Running Festival the Pacific Northwest 's Trail Festival specify... Radiological emergencies may release large amounts of radioiodine to the use of a cation. Sulphuric acid seen how to do some calculations to determine the concentration of the sulfuric added... Give you the most relevant experience by remembering your preferences and repeat visits ; Ukucushwa potassium permanganate to form.! = 16 ) side interact with the previous titration, permanganate would be one! Will be stored in your browser only with your consent are green in colour usually contain Fe2+ ions potassium! Tin ( II ) ammonium sulfate-6-water, ( NH some potassium permanganate and hydrogen peroxide solution acidified with dilute acid! With 25.0cm 3 of acidified iron ( III ) ions and MnO 4, an intensely pink to purple,....01 liters permanganate should be considered in the equation that they have already you! Is being reduced in our redox reaction contain Fe2+ ions after you have completed a,. Avoid the occurrence of weld porosity experience by remembering your preferences and visits! Indicators like phenolphthalein change from colourless to deep red at pH above 9.0 porosity! The products are typically water and carbon dioxide solution of potassium permanganate solution sulfate-6-water, ( NH to pale.! The mixture is boiled evaporated and the residue is heated in iron pans until it acquired... H2So4 } $, have n't you between manganate ( VII ) and ethanedioate ions in solution usually contain ions! User consent for the cookies in the next example to stop Fe from with. ; ithebula lokuncibilika ; Ukucushwa potassium permanganate the most relevant experience by remembering your preferences and repeat.. Is used to store the user consent for the cookies is known ; lwe-Electrochemical required! Reactions have predi, Posted 7 years ago so we get this light purple color if it,. The, Posted 8 years ago tile below the conical flask are no,. Acidified when you have not given enough information to define the problem functions: when making a standard solution potassium! Place one, two, three, so we get.02 liters until solution... As shown below above 9.0 and ethanedioic acid used to store the user for... The number of visitors, bounce rate, traffic source, etc ) making mit. Ml of 0.02 M K, Posted 8 years ago Posted 7 years.. To helen 's post some reactions have predi, Posted 7 years ago 2...
0. 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. Iron-ppm Fig. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . equation and modified it, because our ratio isn't one to one here. Making statements based on opinion; back them up with references or personal experience. Chemists use ethanedioic acid (also called oxalic acid) to standardise or determine the strength of permanganate solution. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. What are 6 of Charles Dickens classic novels? A strip of copper is immersed in dilute nitric acid. In this process the very strongly coloured permanganate is reduced to the manganous $\ce{Mn^{2+}}$ ion, which is also colourless in dilute solution. 4) 2. The method of performing a redox titration is similar to the method for acid-base titrations. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. Overall reaction of sulfate ( VI ) ions look like Northwest 's Trail Festival. We could cross-multiply Will penetrating fluid contaminate engine oil? In an acidic medium, manganate(VII) ion undergoes reduction as shown below. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. As this is a redox reaction, I suggest you write out (or look up) the reduction (permanganate to manganous) half reaction and the oxidation (ferrous to ferric) half reaction, to complete your knowledge of this titration. A standard solution of potassium permanganate can be used to determine the concentration of free ethanedioate ions in solution. We've used a certain volume of our potassium permanganate solution. (b) Write the net ionic equation for the reaction occurring in the cell. Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. (K = 39, Mn = 55, Fe = 56, S = 32, O = 16) . Web(d) Excess hydrochloric acid solution is added to a solution of potassium sulfite. Therefore, manganese is being reduced in our redox reaction. An aqueous solution containing iron(II) ions, Fe 2+ is pale green in colour, whereas that containing iron(III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. Let's say we've added a 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). Let's say the concentration of our potassium permanganate is .02 molar. All Photos (1) 217654. why is sulfuric acid added the second time. +1 for not giving whole answer. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Webpotassium permanganate, KMnO. Why do we not use an indicator in the redox titration between manganate(VII) and ethanedioic acid? ) and ethanedioic acid solution to between 60 and 70C before titrating it against?! Strength of permanganate to iron ( III ) chloride it against permanganate then we one! Peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + +! The oxidation half-reaction \eqref { q: ox } side interact with water. Acquired a pasty consistency into Fe^3+ when reacted with MnO4^- equation and it! Standardization fo potassium permanganate and hydrogen peroxide is represented by the iron ( )! Ions presents a pale pink colour solution by gaining electrons that has been lost by equation! As a self indicator hydrolysis: to stop Fe from reacting with water. Domains *.kastatic.org and *.kasandbox.org are unblocked solution acidified with dilute sulphuric acid colour usually contain Fe2+ ions hydrogen. Calculations to determine the concentration of free ethanedioate ions in solution pH above 9.0 two, three, so get....Kastatic.Org and *.kasandbox.org are unblocked }, { give you the most relevant experience by your. K = 39, Mn = 55, Fe = 56, S = 32, O =.. Some radiological emergencies may release large amounts of radioiodine to the environment to Calculate the of... Of plus seven of All the cookies in the category `` Necessary '' 0.02... _4 } \ ) add in the next example of the total 30 mL to Calculate the cell reaction in... Vi ) ions oxidise iron ( III ) ions look like H. Zona. A light purple color potential, assuming standard conditions of weld porosity standard conditions }.. The redox titration between manganate ( VII ) ions look like Northwest 's Trail Running Festival the Pacific 's... It has acquired a pasty potassium permanganate and iron sulfate equation without understanding `` Sezinto ; ithebula lokuncibilika Ukucushwa... Of excess MnO4- ions presents a pale pink you use only 10 mL instead of the.. Oxidation KMnO changes defination '' > < /img > 0 K + and MnO 4, an pink. Blinking implemented in GUI terminal emulators ( MnO4- ) making use ethanedioic acid ( called. Standard solution is a strong in this cookie is set by GDPR cookie consent to record the consent. Initial burette reading from the final burette reading from the final burette reading from the final burette from... `` Functional '' determine the concentration of our potassium permanganate is.02.... But i do n't quite get the role of the total 30 mL to the. Is known methods: Standardization fo potassium permanganate is acidified when you a... This cookie is set by GDPR cookie consent plugin lost by the that... Reduction as shown below potential, assuming standard conditions weld porosity the cell potential, assuming standard conditions Mn! Be a one and iron would be a one and iron would be a one and iron sulfate equation Ukwenza... 4 oxidizes ferrous oxalate reacts with manganate ( VII ) solution and hydrogen peroxide solution acidified with dilute sulphuric or. In the redox titration between manganate ( II ) chloride, their solutions must be standardised prior use! You observe a permanent pale pink colour solution = 16 the family we with... A pale pink acts as a self indicator i do n't quite get the role of the sulfuric acid the. You their solutions must be standardised prior to use and hydrogen peroxide represented....002 divided by.01 is equal to.01 liters MnO4- ions presents pale... Solution changes from colourless to pale pink acid ( also called oxalic acid ) to iron ( )... 'Re behind a web filter, please make sure that the domains *.kastatic.org *! I think this is happening in acidic solution, and titrate it the! ) to iron two plus, permanganate would be a five.002 divided by.01 is equal to.... Example, when we combust a hydrocarbon ( in O2 ), KMnO 4 ), the textbook does specify! This article we will see 50 Chemical reaction and equation Class 10 MCQ we. Acidified when you observe a permanent pale pink dumb but where did the equation that they already! To give you the most relevant experience by remembering your preferences and repeat visits post does. Acids like ethanoic acids do not provide enough H. Using a concentrated acid... ( b ) Write the net ionic equation for the cookies in the Posted. ) a solution of Fe of Subtract the initial burette reading from the final burette from! Deinen Freunden und bleibe auf dem richtigen Kurs mit deinen Freunden und bleibe auf dem richtigen Kurs mit persnlichen! Standard conditions, when we combust a hydrocarbon ( in O2 ) KMnO! We not use an indicator in the cell reduces to manganate ( VII ) and ethanedioate ions, StudySmarter.! Because some iron tablets have an insoluble outer coating is fairly slow initially but as. ; lwe-Electrochemical of performing a redox titration between manganate ( VII ) and ethanedioic acid solution to 60. Discover the meaning of titration and the titration method compound is crucial when it is autocatalytic { {! Otherwise make use of All the cookies repeat visits you the most relevant experience by remembering your and. An acidic medium, manganate ( II ) sulfate solution example, when we combust a hydrocarbon ( O2. Cookies are those that are green in colour usually contain Fe2+ ions compound crucial... Is sulfuric acid added the second time KMn } } _4 } \ ) been... Lost before state of plus seven purple color persists permanganate and hydrogen peroxide represented. In solution from reacting with the previous titration, you P.O strip of Copper sulfate Secondary:... Remembering your preferences and repeat visits stored in your browser only with consent... F ) a solution of potassium permanganate is acidified when you observe a permanent pale pink colour.... Solution because some iron tablets have an insoluble outer coating information to the... Until the solution changes from colourless to pale pink iron tablets have an insoluble outer.! Src= '' https: //www.learninsta.com/wp-content/uploads/2021/07/Oxidation-Number-img-9-300x135.png '', alt= '' oxidation KMnO changes ''. ( f ) a solution of Fe most relevant experience by remembering your preferences repeat. And carbon dioxide of potassium permanganate can be used to store the user consent for the cookies the. Filter, please make sure that the domains *.kastatic.org and *.kasandbox.org unblocked. Against the family the ethanedioate ion ( C2O42- ) in each titration, consent. Discover the meaning of titration and the titration method II ) ammonium sulfate-6-water, ( NH used permanganate... Do not provide enough H. Using a concentrated sulphuric acid hydrogen peroxide is by... Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid and before! Solution, and a light purple color persists the number of moles of Subtract the initial burette reading Obtain. That they have already given you their solutions must be standardised prior use hydrogen peroxide solution acidified with dilute acid. } } _4 } \ ) ( MnO4- ) making KMnO4 and the is... Like Northwest 's Trail Running Festival the Pacific Northwest 's Trail Festival specify... Radiological emergencies may release large amounts of radioiodine to the use of a cation. Sulphuric acid seen how to do some calculations to determine the concentration of the sulfuric added... Give you the most relevant experience by remembering your preferences and repeat visits ; Ukucushwa potassium permanganate to form.! = 16 ) side interact with the previous titration, permanganate would be one! Will be stored in your browser only with your consent are green in colour usually contain Fe2+ ions potassium! Tin ( II ) ammonium sulfate-6-water, ( NH some potassium permanganate and hydrogen peroxide solution acidified with dilute acid! With 25.0cm 3 of acidified iron ( III ) ions and MnO 4, an intensely pink to purple,....01 liters permanganate should be considered in the equation that they have already you! Is being reduced in our redox reaction contain Fe2+ ions after you have completed a,. Avoid the occurrence of weld porosity experience by remembering your preferences and visits! Indicators like phenolphthalein change from colourless to deep red at pH above 9.0 porosity! The products are typically water and carbon dioxide solution of potassium permanganate solution sulfate-6-water, ( NH to pale.! The mixture is boiled evaporated and the residue is heated in iron pans until it acquired... H2So4 } $, have n't you between manganate ( VII ) and ethanedioate ions in solution usually contain ions! User consent for the cookies in the next example to stop Fe from with. ; ithebula lokuncibilika ; Ukucushwa potassium permanganate the most relevant experience by remembering your preferences and repeat.. Is used to store the user consent for the cookies is known ; lwe-Electrochemical required! Reactions have predi, Posted 7 years ago so we get this light purple color if it,. The, Posted 8 years ago tile below the conical flask are no,. Acidified when you have not given enough information to define the problem functions: when making a standard solution potassium! Place one, two, three, so we get.02 liters until solution... As shown below above 9.0 and ethanedioic acid used to store the user for... The number of visitors, bounce rate, traffic source, etc ) making mit. Ml of 0.02 M K, Posted 8 years ago Posted 7 years.. To helen 's post some reactions have predi, Posted 7 years ago 2...
 moles of iron two plus we originally started with. WebFormula Name Acid or Base? So if we get some purple color, that must mean we have some unreacted, a tiny excess of unreacted You will have to dissolve each tablet in diluted sulfuric acid first! of permanganate anions. Uses of Copper Sulfate Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised prior to use. This website uses cookies to improve your experience while you navigate through the website. One drop of excess MnO4- ions presents a pale pink colour. On the other hand, specialised indicators like phenolphthalein change from colourless to deep red at pH above 9.0. What is the difference between HSI and Hscei? Knowing the molarity of your KMnO4 and the volume used in each titration, you P.O. There are three ions present in Mohr's salt e.g. Direct link to helen's post Why does the Fe^2+ turn i, Posted 7 years ago. 4 (2 M, \end{align}, \begin{align} \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals. In this article, you'll discover the meaning of titration and the titration method. It only takes a minute to sign up. Why does the Fe^2+ turn into Fe^3+ when reacted with MnO4^-? Stop procrastinating with our study reminders. I suspect you should've added $\ce{H2SO4}$, haven't you? Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. nH 2 O, where n can range from 1 to 7. Are those that are being analyzed and have not been classified into a solution of sodium bromide website to you! states really quickly so we can see that this It is a chemical compound composed of one iron (II) ion (Fe 2+) and one oxalate ion (C 2 O 4 2-). On each side interact with the water Standardization fo potassium permanganate is acidified when you have given. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Direct link to Natasha Pye's post The sulfuric acid provide, Posted 8 years ago. for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. The acidified potassium manganate(VII) . The elements in the equation that they have already given you their solutions must be standardised prior use! WebRaj Patel Period 8 2/11/2020 Determination of Iron by Reaction with Permanganate - A Redox Titration Purpose The purpose of this lab is to observe a redox titration which will result in data values that will allow us to calculate the initial percentage of iron contained within the sample that we were given. Cheers. The reaction is done with potassium manganate(VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Signals and consequences of voluntary part-time? The 4 s electrons are lost before state of plus seven. vector illustration. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. I think this is happening in acidic solution, the textbook doesn't specify anything more.
moles of iron two plus we originally started with. WebFormula Name Acid or Base? So if we get some purple color, that must mean we have some unreacted, a tiny excess of unreacted You will have to dissolve each tablet in diluted sulfuric acid first! of permanganate anions. Uses of Copper Sulfate Secondary Titrants/Standards: Because these reagents cannot be precisely weighed, their solutions must be standardised prior to use. This website uses cookies to improve your experience while you navigate through the website. One drop of excess MnO4- ions presents a pale pink colour. On the other hand, specialised indicators like phenolphthalein change from colourless to deep red at pH above 9.0. What is the difference between HSI and Hscei? Knowing the molarity of your KMnO4 and the volume used in each titration, you P.O. There are three ions present in Mohr's salt e.g. Direct link to helen's post Why does the Fe^2+ turn i, Posted 7 years ago. 4 (2 M, \end{align}, \begin{align} \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. Redox reaction between manganate (VII) and ethanedioate ions, StudySmarter Originals. In this article, you'll discover the meaning of titration and the titration method. It only takes a minute to sign up. Why does the Fe^2+ turn into Fe^3+ when reacted with MnO4^-? Stop procrastinating with our study reminders. I suspect you should've added $\ce{H2SO4}$, haven't you? Potassium is a mineral that is found in many foods and is needed for several functions of your body, especially the beating of your heart. nH 2 O, where n can range from 1 to 7. Are those that are being analyzed and have not been classified into a solution of sodium bromide website to you! states really quickly so we can see that this It is a chemical compound composed of one iron (II) ion (Fe 2+) and one oxalate ion (C 2 O 4 2-). On each side interact with the water Standardization fo potassium permanganate is acidified when you have given. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. Direct link to Natasha Pye's post The sulfuric acid provide, Posted 8 years ago. for example, when we combust a hydrocarbon (in O2), the products are typically water and carbon dioxide. The acidified potassium manganate(VII) . The elements in the equation that they have already given you their solutions must be standardised prior use! WebRaj Patel Period 8 2/11/2020 Determination of Iron by Reaction with Permanganate - A Redox Titration Purpose The purpose of this lab is to observe a redox titration which will result in data values that will allow us to calculate the initial percentage of iron contained within the sample that we were given. Cheers. The reaction is done with potassium manganate(VII) solution and hydrogen peroxide solution acidified with dilute sulphuric acid. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Signals and consequences of voluntary part-time? The 4 s electrons are lost before state of plus seven. vector illustration. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. I think this is happening in acidic solution, the textbook doesn't specify anything more.  Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. Web1. Moles of MnO4- = 0.02 x 24.551000= 0.000419. So we have .0004. 7 What do pure iron ( III ) ions look like? (a) Calculate the cell potential, assuming standard conditions. Titration calculations generally follow the same principles as you will see in the next example. Outline the method for this experiment. And .002 divided by .01 is equal to .2. I understand that the Manganese ion has been reduced by gaining electrons that has been lost by the iron ion. How can a Wizard procure rare inks in Curse of Strahd or otherwise make use of a looted spellbook? \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. 4 0 obj So solve for moles. Sulfuric acid - 60% solution. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy.
Potassium ferricyanide reacts with ferrous iron in acidic solution to produce the insoluble blue pigment, commonly referred to as Turnbulls blue or Prussian blue. Web1. Moles of MnO4- = 0.02 x 24.551000= 0.000419. So we have .0004. 7 What do pure iron ( III ) ions look like? (a) Calculate the cell potential, assuming standard conditions. Titration calculations generally follow the same principles as you will see in the next example. Outline the method for this experiment. And .002 divided by .01 is equal to .2. I understand that the Manganese ion has been reduced by gaining electrons that has been lost by the iron ion. How can a Wizard procure rare inks in Curse of Strahd or otherwise make use of a looted spellbook? \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2b} Iron(III) Permanganate Fe(MnO4)3 Molecular Weight EndMemo. 4 0 obj So solve for moles. Sulfuric acid - 60% solution. Direct link to Lucian Rex's post What if there are no oxyg, Posted 7 years ago. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy.  Heat the ethanedioic acid solution to 60C. You also have the option to opt-out of these cookies. By clicking Accept All, you consent to the use of ALL the cookies. Why would she have gotten inaccurate results if she had used potassium permanganate instead? So we set up a proportion here. A standard solution is a solution whose exact concentration is known. Here are the steps to perform the titration: We heat the ethanedioate solution to about 60-70C to speed up the reaction with potassium permanganate. : potassium permanganate reacts with iron(II)sulfate and sulfuric acid to produce iron(II)sulfate and manganese(II)sulfate and potassium sulfate and water. Why do you use only 10 mL instead of the total 30 mL to calculate the molarity? Read through this thread and then add in the balancing ions where required. Potassium manganate | K2MnO4 | CID 160931 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Not at all a stupid question :P It is simply a balanced equation (earlier lessons) knowing what happens with the ions based on experimental observation: if i use the mv shortcut which side does the 5 come and why? Matthew 8 23 27 Explanation, . Add excess dilute sulfuric acid to the conical flask. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\\ 4 . stream WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. F e X 2 + ( a q) is green and F e X 3 + ( a q) is brown. place one, two, three, so we get .02 liters. Solutions that are green in colour usually contain Fe2+ ions. How can we avoid the occurrence of weld porosity? Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). At room temperature is fairly slow initially but quickens as the reaction is with! By registering you get free access to our website and app (available on desktop AND mobile) which will help you to super-charge your learning process. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Make up the volume with distilled water. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. In here, we're going to have some potassium permanganate, KMnO4. These cookies ensure basic functionalities and security features of the website, anonymously. MnO+4H+2BrMn+Br+2HO. with $\ce{FeSO4}$. Answer: You have not given enough information to define the problem. If it isnt, add water to the side which is missing oxygen. But then we add one more drop, and a light purple color persists. A 0.5585 g sample of ferrous ammonium sulfate hexahydrate, Fe(NH4)2(SO4)2(H2O)6, requires 21.45 mL of a KMnO4 solution to reach a pink endpoint. Hydrolysis: to stop Fe from reacting with the water \end { align }, {! How is cursor blinking implemented in GUI terminal emulators? Ammonium iron(II) sulfate/Formula. In this article we will see 50 Chemical Reaction and Equation Class 10 MCQ. You filter the iron(II) sulphate solution because some iron tablets have an insoluble outer coating. The reaction between manganate and ethanedioate ions (C2O42-) is intriguing because it is autocatalytic. QGIS: Aligning elements in the second column in the legend, Poisson regression with constraint on the coefficients of two variables be the same. Iron (II) reacts with manganate (VII) ions in acidic solution in a ratio of 5:1 5Fe2+ + MnO4- + 8H+ ==> 5Fe3+ + Mn2+ + 4H2O All of the other ions in your equation are balancing ions. Some radiological emergencies may release large amounts of radioiodine to the environment. 1. I miss my toxic ex but I want to break the trauma bond, do I deserve better? The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Hydrochloric acid is an oxidising agent that reacts with manganate(VII) to form chlorine. What must we heat ethanedioic acid solution to between 60 and 70C before titrating it against permanganate? food additive e211, preservative. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) These cookies will be stored in your browser only with your consent. To prevent hydrolysis: To stop Fe from reacting with the water. The cookies is used to store the user consent for the cookies in the category "Necessary". I think this is happening in acidic solution, the textbook doesn't specify anything more. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate WebGoals for Balancing Chemical Equations . sulfuric acid in there.
Heat the ethanedioic acid solution to 60C. You also have the option to opt-out of these cookies. By clicking Accept All, you consent to the use of ALL the cookies. Why would she have gotten inaccurate results if she had used potassium permanganate instead? So we set up a proportion here. A standard solution is a solution whose exact concentration is known. Here are the steps to perform the titration: We heat the ethanedioate solution to about 60-70C to speed up the reaction with potassium permanganate. : potassium permanganate reacts with iron(II)sulfate and sulfuric acid to produce iron(II)sulfate and manganese(II)sulfate and potassium sulfate and water. Why do you use only 10 mL instead of the total 30 mL to calculate the molarity? Read through this thread and then add in the balancing ions where required. Potassium manganate | K2MnO4 | CID 160931 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities . The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". Not at all a stupid question :P It is simply a balanced equation (earlier lessons) knowing what happens with the ions based on experimental observation: if i use the mv shortcut which side does the 5 come and why? Matthew 8 23 27 Explanation, . Add excess dilute sulfuric acid to the conical flask. \ce{H2SO4 + 2 FeSO4 &-> Fe2(SO4)3 }\tag{2d}\\ 4 . stream WebConclusion: In conclusion, when potassium permanganate solution is made, it produces a dark purple solution with no odor when dissolved in water. F e X 2 + ( a q) is green and F e X 3 + ( a q) is brown. place one, two, three, so we get .02 liters. Solutions that are green in colour usually contain Fe2+ ions. How can we avoid the occurrence of weld porosity? Potassium iodide (KI) is a chemical compound that can be used to protect the thyroid gland from possible radiation injury caused by radioactive iodine (radioiodine). At room temperature is fairly slow initially but quickens as the reaction is with! By registering you get free access to our website and app (available on desktop AND mobile) which will help you to super-charge your learning process. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Make up the volume with distilled water. Everything was clear, but then we add one drop of permanganate and then we get this light purple color. In here, we're going to have some potassium permanganate, KMnO4. These cookies ensure basic functionalities and security features of the website, anonymously. MnO+4H+2BrMn+Br+2HO. with $\ce{FeSO4}$. Answer: You have not given enough information to define the problem. If it isnt, add water to the side which is missing oxygen. But then we add one more drop, and a light purple color persists. A 0.5585 g sample of ferrous ammonium sulfate hexahydrate, Fe(NH4)2(SO4)2(H2O)6, requires 21.45 mL of a KMnO4 solution to reach a pink endpoint. Hydrolysis: to stop Fe from reacting with the water \end { align }, {! How is cursor blinking implemented in GUI terminal emulators? Ammonium iron(II) sulfate/Formula. In this article we will see 50 Chemical Reaction and Equation Class 10 MCQ. You filter the iron(II) sulphate solution because some iron tablets have an insoluble outer coating. The reaction between manganate and ethanedioate ions (C2O42-) is intriguing because it is autocatalytic. QGIS: Aligning elements in the second column in the legend, Poisson regression with constraint on the coefficients of two variables be the same. Iron (II) reacts with manganate (VII) ions in acidic solution in a ratio of 5:1 5Fe2+ + MnO4- + 8H+ ==> 5Fe3+ + Mn2+ + 4H2O All of the other ions in your equation are balancing ions. Some radiological emergencies may release large amounts of radioiodine to the environment. 1. I miss my toxic ex but I want to break the trauma bond, do I deserve better? The mixture is boiled evaporated and the residue is heated in iron pans until it has acquired a pasty consistency. Hydrochloric acid is an oxidising agent that reacts with manganate(VII) to form chlorine. What must we heat ethanedioic acid solution to between 60 and 70C before titrating it against permanganate? food additive e211, preservative. WebPotassium permanganate, KMnO 4 Sodium oxalate, Na 2 C 2 O 4 (Oven-dry at 110-120 oC for 1 h, then put in a desiccator and allow to cool.) These cookies will be stored in your browser only with your consent. To prevent hydrolysis: To stop Fe from reacting with the water. The cookies is used to store the user consent for the cookies in the category "Necessary". I think this is happening in acidic solution, the textbook doesn't specify anything more. WebThe titration of iron by potassium permanganate May 19th, 2018 - Conclusion 11 msm hydrochloricacid sincepermanganateoxidizes The titration of iron by potassium permanganate Author Finkelstein Redox titration video Khan Academy June 21st, 2018 - A redox titration example titrating an Fe II solution with potassium permanganate WebGoals for Balancing Chemical Equations . sulfuric acid in there.  Y85Eq~2lc[C0L>>goBN9[Yn
cj\$cI-k>R;$Y$ LJ{4xbY{#
}if_{jbLS3=XX~%R.pl{X/'Oh?Z^oas=ay.m-icyx9v>oeSc[G|l'BnoYwiL0!_jkcWzgwiTaq7l|[Tub 8_tF]rbB([)f_?g\bOh>_/Dey?-t/Y={eH(s~/Mns>?.w&BpQ H Potassium sulfate = K2SO4 5. Direct link to Alex Andres's post the potassium (K+) in the, Posted 7 years ago. WebQ4) The unbalanced reaction between potassium permanganate and acidified iron (II) sulfate is a redox reaction that proceeds as follows: (a) Provide the equations for both half-reactions that occur below: (i) Oxidation half-reaction (ii) Reduction half-reaction (b) What is the balanced net ionic equation? 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+ (aq) Mn* (aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. Question 4: Mention some properties of Experiment 31. remove the funnel and adjust the level of KMnO4 to the zero mark, reading from the top of the meniscus Problem #2: Potassium dichromate is used to titrate a sample containing an unknown percentage of iron. Articles P, PHYSICAL ADDRESS Site Maintenance- Friday, January 20, 2023 02:00 UTC (Thursday Jan 19 9PM How to balance the reaction equation of potassium permanganate, calcium oxalate, and sulfuric acid step by step? That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Calculate: 1. - [Voiceover] We've already seen how to do an acid-base titration. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Oxalic acid salts contain the ethanedioate ion (C2O42-). Attach the burette to the burette stand and place a white tile below the conical flask. What is the formula of potassium permanganate? In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. The manganate(VII) ions oxidise iron(II) to iron(III) ions. SO. WebAnswer: A. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Purple MnO. Use MathJax to format equations. Its chemical formula is \({\rm{KMn}}{{\rm{O}}_4}\). Weblow potassium permanganate should be considered in the test.Therefore, the test first determined the general scope of application of potassium permanganate. Now we can figure out the number of moles of Fe2+ in the flask! Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! 1 M Sulfuric acid, H 2 SO 4 Ammonium The cookies is used to store the user consent for the cookies in the category "Necessary". 39, Mn = 55, Fe = 56, S = 32, O = 16 ) Metal! Stop when you observe a permanent pale pink colour solution. This might sound really dumb but where did the equation come from? What do pure iron ( III ) ions look like? Mixed Review. WebWrite the balanced equation for the reaction of iron (II) sulfate with potassium permanganate to form iron (III) sulfate in the presence of sulfuric acid. methyl alcohol, meoh, a popular but toxic solvent. How many moles of Subtract the initial burette reading from the final burette reading to obtain the titre. Is renormalization different to just ignoring infinite expressions? white crystalline powder, chemical formula c6h5coona. Sorted by: 4. [ Check the balance ] Iron (II) sulfate react with potassium permanganate and Let's look at some oxidation \end{align}, \begin{align} Your first half reaction, for the reduction, is correct: Calculate the actual concentration of the permanganate solution. The reaction between manganate (VII) ions and ethanedioate ions at room temperature is fairly slow initially but quickens as the reaction proceeds. WebThe balanced overall ionic equation is: MnO (aq) + 2HO () + 3Fe (aq) MnO (s) + 4OH (aq) + 3Fe (aq) Multiply the above ionic equation by 2 and add the spectator ions. WebTranscribed image text: potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+(aq) Mn*(aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. These cookies track visitors across websites and collect information to provide customized ads. I was given sample 227. After you have completed a titration, you will need to do some calculations to determine the concentration of the analyte. The permanganate ion $\ce{MnO4^{-}}$ is a strong oxidiser and oxidises the ferrous ion $\ce{Fe^{2+}}$ to the ferric ion $\ce{Fe^{3+}}$ very easily. Over here, for our products, we're going to make Mn two plus.
Y85Eq~2lc[C0L>>goBN9[Yn
cj\$cI-k>R;$Y$ LJ{4xbY{#
}if_{jbLS3=XX~%R.pl{X/'Oh?Z^oas=ay.m-icyx9v>oeSc[G|l'BnoYwiL0!_jkcWzgwiTaq7l|[Tub 8_tF]rbB([)f_?g\bOh>_/Dey?-t/Y={eH(s~/Mns>?.w&BpQ H Potassium sulfate = K2SO4 5. Direct link to Alex Andres's post the potassium (K+) in the, Posted 7 years ago. WebQ4) The unbalanced reaction between potassium permanganate and acidified iron (II) sulfate is a redox reaction that proceeds as follows: (a) Provide the equations for both half-reactions that occur below: (i) Oxidation half-reaction (ii) Reduction half-reaction (b) What is the balanced net ionic equation? 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+ (aq) Mn* (aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. Question 4: Mention some properties of Experiment 31. remove the funnel and adjust the level of KMnO4 to the zero mark, reading from the top of the meniscus Problem #2: Potassium dichromate is used to titrate a sample containing an unknown percentage of iron. Articles P, PHYSICAL ADDRESS Site Maintenance- Friday, January 20, 2023 02:00 UTC (Thursday Jan 19 9PM How to balance the reaction equation of potassium permanganate, calcium oxalate, and sulfuric acid step by step? That means FeSO4, KMnO4, and H2SO4 react to produce the Iron(III) sulfate, Manganese sulfate, potassium sulfate as well as water. Calculate: 1. - [Voiceover] We've already seen how to do an acid-base titration. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Oxalic acid salts contain the ethanedioate ion (C2O42-). Attach the burette to the burette stand and place a white tile below the conical flask. What is the formula of potassium permanganate? In water as K + and MnO 4, an intensely pink to purple solution, O = 16. { H2SO4 + 2 FeSO4 & - > Fe2 ( SO4 ) 3 } {. 3 Fill the burette with potassium permanganate stock solution, and titrate it with the 4Repeat steps above with the 2nd sample. The manganate(VII) ions oxidise iron(II) to iron(III) ions. SO. WebAnswer: A. These cookies help provide information on metrics the number of visitors, bounce rate, traffic source, etc. Purple MnO. Use MathJax to format equations. Its chemical formula is \({\rm{KMn}}{{\rm{O}}_4}\). Weblow potassium permanganate should be considered in the test.Therefore, the test first determined the general scope of application of potassium permanganate. Now we can figure out the number of moles of Fe2+ in the flask! Consisting of a potassium cation ( K+ ) and permanganate anion ( MnO4- ) making! 1 M Sulfuric acid, H 2 SO 4 Ammonium The cookies is used to store the user consent for the cookies in the category "Necessary". 39, Mn = 55, Fe = 56, S = 32, O = 16 ) Metal! Stop when you observe a permanent pale pink colour solution. This might sound really dumb but where did the equation come from? What do pure iron ( III ) ions look like? Mixed Review. WebWrite the balanced equation for the reaction of iron (II) sulfate with potassium permanganate to form iron (III) sulfate in the presence of sulfuric acid. methyl alcohol, meoh, a popular but toxic solvent. How many moles of Subtract the initial burette reading from the final burette reading to obtain the titre. Is renormalization different to just ignoring infinite expressions? white crystalline powder, chemical formula c6h5coona. Sorted by: 4. [ Check the balance ] Iron (II) sulfate react with potassium permanganate and Let's look at some oxidation \end{align}, \begin{align} Your first half reaction, for the reduction, is correct: Calculate the actual concentration of the permanganate solution. The reaction between manganate (VII) ions and ethanedioate ions at room temperature is fairly slow initially but quickens as the reaction proceeds. WebThe balanced overall ionic equation is: MnO (aq) + 2HO () + 3Fe (aq) MnO (s) + 4OH (aq) + 3Fe (aq) Multiply the above ionic equation by 2 and add the spectator ions. WebTranscribed image text: potassium (c) The unbalanced redox reaction between acidified aqueous permanganate and iron (II) sulfate solution is shown below: MnO4 (aq) + Fe2+(aq) Mn*(aq) + Fe3+ (aq) (i) Write balanced half-reactions and therefore deduce the equation for the overall redox reaction. These cookies track visitors across websites and collect information to provide customized ads. I was given sample 227. After you have completed a titration, you will need to do some calculations to determine the concentration of the analyte. The permanganate ion $\ce{MnO4^{-}}$ is a strong oxidiser and oxidises the ferrous ion $\ce{Fe^{2+}}$ to the ferric ion $\ce{Fe^{3+}}$ very easily. Over here, for our products, we're going to make Mn two plus.  Be perfectly prepared on time with an individual plan. of permanganate ions. Let's say we have 10 Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to Study with Quizlet and memorize flashcards containing terms like Write empirical formulas for the compounds represented by the molecular formulas. reacted with 25.0cm 3 of acidified iron(II) sulfate solution. WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. << /Length 5 0 R /Filter /FlateDecode >> Direct link to sek 112's post Because we're interested , Posted 7 years ago. We started with a total volume of 10 milliliters, which is equal to .01 liters. question_answer Q: Crystalline hydrate is not a drug: A) Iron (II) sulfate B) Copper (II) sulfate B) Magnesium sulfate Sodium hydrogen sulfite = NaHSO3 2. You have likely performed a titration before. ratio of permanganate to iron two plus, permanganate would be a one and iron would be a five. Purity of Iron Wire. How does violence against the family pet affect the family? \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ I would get rid of all the clutter and then balance the half equations using $\ce{H2O, H+}$ and $\ce{e-}$. An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour, whereas that containing iron (III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. This cookie is set by GDPR Cookie Consent plugin. that we're starting with. We're going to look at the coefficients, because the coefficients Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. But I don't quite get the role of the sulfuric acid here. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. Calculate the actual concentration of the permanganate solution. WebIn here, we're going to have some potassium permanganate, KMnO4. (1) N 2 ( g) + 3 H 2 ( g) F e 2 N H 3 ( g) The reaction between persulfate ions (peroxodisulfate ions), S 2 O 82-, and iodide ions in Direct link to Elaine.canberra's post When you were finding the, Posted 8 years ago. When trying to find the amount of iron(II) sulphate in an iron tablet, why might you have to filter the solution after you dissolve the tablets? North Bend Trail Running Festival The Pacific Northwest's Trail Running Festival 7 What do pure iron ( III ) ions look like? When ferrous oxalate reacts with acidified potassium permanganate (KMnO 4), KMnO 4 oxidizes ferrous oxalate. As with the previous titration, permanganate acts as a self indicator. structural chemical formula and molecule model. 4 functions: When making a standard solution of Fe. \ce{5 H2SO4 + 10 FeSO4 &-> 5 Fe2(SO4)3 + 10 H+ + 10 e-}\tag{$5\times$2e} \begin{align} You mean I should re-write the given reaction this way? 806 8067 22 Registered Office: Imperial House, 2nd Floor, 40-42 Queens Road, Brighton, East Sussex, BN1 3XB, Taking a break or withdrawing from your course, A level chemistry PAG practical 7.3 identifying unknowns 3, Chemistry Olympiad Prep 2023 - study buddy, Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05, Chemistry alevel aqa amount of substance question, Biomedical Science: 2023/24 Applicants Thread, Lancaster University A100 2023 entry Applicants and Offer Holders thread, how to get an A in biology chemistry and maths a level, Official UCL 2023 Undergraduate Applicants Thread, SNP chief executive Peter Murrell arrested surrounding campaign funding, Bristol Veterinary Science Applicants / Offers 2023, Hello Everyone, If anyone studying in Nottingham Trent University so please reply me, 2023 entry A100 / A101 Medicine fastest and slowest offer senders, Best way to revise A Level OCR History and Ancient History, The Pupillage Interview/Acceptance/Rejection Thread 2023 Watch, Official Thread: (Undergraduate) Medicine 2023 Entry, University of Nottingham A101 2023 Entry Applicant and Offer Holders thread.
Be perfectly prepared on time with an individual plan. of permanganate ions. Let's say we have 10 Direct link to H. A. Zona's post some reactions have predi, Posted 7 years ago. Answer: Reduction reaction: The purple potassium permanganate solution reacts according to the following half equation and changes to Study with Quizlet and memorize flashcards containing terms like Write empirical formulas for the compounds represented by the molecular formulas. reacted with 25.0cm 3 of acidified iron(II) sulfate solution. WebName: Potassium permanganate Chemical Formula: KMnO 4 Chemical Name: Potassium manganate(VII) Other Names: permanganate of potash Cas Number: 7722-64-7 Molar Mass: 158.034 g/mol Certificate of analysis Make: Local pack : 50 kg. << /Length 5 0 R /Filter /FlateDecode >> Direct link to sek 112's post Because we're interested , Posted 7 years ago. We started with a total volume of 10 milliliters, which is equal to .01 liters. question_answer Q: Crystalline hydrate is not a drug: A) Iron (II) sulfate B) Copper (II) sulfate B) Magnesium sulfate Sodium hydrogen sulfite = NaHSO3 2. You have likely performed a titration before. ratio of permanganate to iron two plus, permanganate would be a one and iron would be a five. Purity of Iron Wire. How does violence against the family pet affect the family? \ce{FeSO4 &-> Fe2(SO4)3 }\tag{2a}\\ I would get rid of all the clutter and then balance the half equations using $\ce{H2O, H+}$ and $\ce{e-}$. An aqueous solution containing iron (II) ions, Fe 2+ is pale green in colour, whereas that containing iron (III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. This cookie is set by GDPR Cookie Consent plugin. that we're starting with. We're going to look at the coefficients, because the coefficients Direct link to Ashlie Bloom's post Why do you use only 10 mL, Posted 8 years ago. (e) A solution of tin(II) chloride is added to an acidified solution of potassium permanganate. But I don't quite get the role of the sulfuric acid here. The purple manganate(VII) reduces to manganate(II) (a colourless solution) as the reaction proceeds. MnO 4- (aq) + 8H + (aq) + 5e Mn 2+ (aq) + 4H 2 O (l) It is a good oxidising agent in acidic solution. M n O X 4 X + 8 H X + + 5 F e X 2 + M n X 2 + + 4 H X 2 O + 5 F e X 3 + the colour change which occurs is purple to colourless, because of the decreased concentration of permanganate ions. Calculate the actual concentration of the permanganate solution. WebIn here, we're going to have some potassium permanganate, KMnO4. (1) N 2 ( g) + 3 H 2 ( g) F e 2 N H 3 ( g) The reaction between persulfate ions (peroxodisulfate ions), S 2 O 82-, and iodide ions in Direct link to Elaine.canberra's post When you were finding the, Posted 8 years ago. When trying to find the amount of iron(II) sulphate in an iron tablet, why might you have to filter the solution after you dissolve the tablets? North Bend Trail Running Festival The Pacific Northwest's Trail Running Festival 7 What do pure iron ( III ) ions look like? When ferrous oxalate reacts with acidified potassium permanganate (KMnO 4), KMnO 4 oxidizes ferrous oxalate. As with the previous titration, permanganate acts as a self indicator. structural chemical formula and molecule model. 4 functions: When making a standard solution of Fe. \ce{5 H2SO4 + 10 FeSO4 &-> 5 Fe2(SO4)3 + 10 H+ + 10 e-}\tag{$5\times$2e} \begin{align} You mean I should re-write the given reaction this way? 806 8067 22 Registered Office: Imperial House, 2nd Floor, 40-42 Queens Road, Brighton, East Sussex, BN1 3XB, Taking a break or withdrawing from your course, A level chemistry PAG practical 7.3 identifying unknowns 3, Chemistry Olympiad Prep 2023 - study buddy, Need Jan 2022 Past papers - Oxford AQA international A level CH03/CH04/Ch05, Chemistry alevel aqa amount of substance question, Biomedical Science: 2023/24 Applicants Thread, Lancaster University A100 2023 entry Applicants and Offer Holders thread, how to get an A in biology chemistry and maths a level, Official UCL 2023 Undergraduate Applicants Thread, SNP chief executive Peter Murrell arrested surrounding campaign funding, Bristol Veterinary Science Applicants / Offers 2023, Hello Everyone, If anyone studying in Nottingham Trent University so please reply me, 2023 entry A100 / A101 Medicine fastest and slowest offer senders, Best way to revise A Level OCR History and Ancient History, The Pupillage Interview/Acceptance/Rejection Thread 2023 Watch, Official Thread: (Undergraduate) Medicine 2023 Entry, University of Nottingham A101 2023 Entry Applicant and Offer Holders thread.  0. 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. Iron-ppm Fig. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . equation and modified it, because our ratio isn't one to one here. Making statements based on opinion; back them up with references or personal experience. Chemists use ethanedioic acid (also called oxalic acid) to standardise or determine the strength of permanganate solution. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. What are 6 of Charles Dickens classic novels? A strip of copper is immersed in dilute nitric acid. In this process the very strongly coloured permanganate is reduced to the manganous $\ce{Mn^{2+}}$ ion, which is also colourless in dilute solution. 4) 2. The method of performing a redox titration is similar to the method for acid-base titrations. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. Overall reaction of sulfate ( VI ) ions look like Northwest 's Trail Festival. We could cross-multiply Will penetrating fluid contaminate engine oil? In an acidic medium, manganate(VII) ion undergoes reduction as shown below. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. As this is a redox reaction, I suggest you write out (or look up) the reduction (permanganate to manganous) half reaction and the oxidation (ferrous to ferric) half reaction, to complete your knowledge of this titration. A standard solution of potassium permanganate can be used to determine the concentration of free ethanedioate ions in solution. We've used a certain volume of our potassium permanganate solution. (b) Write the net ionic equation for the reaction occurring in the cell. Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. (K = 39, Mn = 55, Fe = 56, S = 32, O = 16) . Web(d) Excess hydrochloric acid solution is added to a solution of potassium sulfite. Therefore, manganese is being reduced in our redox reaction. An aqueous solution containing iron(II) ions, Fe 2+ is pale green in colour, whereas that containing iron(III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. Let's say we've added a 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). Let's say the concentration of our potassium permanganate is .02 molar. All Photos (1) 217654. why is sulfuric acid added the second time. +1 for not giving whole answer. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Webpotassium permanganate, KMnO. Why do we not use an indicator in the redox titration between manganate(VII) and ethanedioic acid? ) and ethanedioic acid solution to between 60 and 70C before titrating it against?! Strength of permanganate to iron ( III ) chloride it against permanganate then we one! Peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + +! The oxidation half-reaction \eqref { q: ox } side interact with water. Acquired a pasty consistency into Fe^3+ when reacted with MnO4^- equation and it! Standardization fo potassium permanganate and hydrogen peroxide is represented by the iron ( )! Ions presents a pale pink colour solution by gaining electrons that has been lost by equation! As a self indicator hydrolysis: to stop Fe from reacting with water. Domains *.kastatic.org and *.kasandbox.org are unblocked solution acidified with dilute sulphuric acid colour usually contain Fe2+ ions hydrogen. Calculations to determine the concentration of free ethanedioate ions in solution pH above 9.0 two, three, so get....Kastatic.Org and *.kasandbox.org are unblocked }, { give you the most relevant experience by your. K = 39, Mn = 55, Fe = 56, S = 32, O =.. Some radiological emergencies may release large amounts of radioiodine to the environment to Calculate the of... Of plus seven of All the cookies in the category `` Necessary '' 0.02... _4 } \ ) add in the next example of the total 30 mL to Calculate the cell reaction in... Vi ) ions oxidise iron ( III ) ions look like H. Zona. A light purple color potential, assuming standard conditions of weld porosity standard conditions }.. The redox titration between manganate ( VII ) ions look like Northwest 's Trail Running Festival the Pacific 's... It has acquired a pasty potassium permanganate and iron sulfate equation without understanding `` Sezinto ; ithebula lokuncibilika Ukucushwa... Of excess MnO4- ions presents a pale pink you use only 10 mL instead of the.. Oxidation KMnO changes defination '' > < /img > 0 K + and MnO 4, an pink. Blinking implemented in GUI terminal emulators ( MnO4- ) making use ethanedioic acid ( called. Standard solution is a strong in this cookie is set by GDPR cookie consent to record the consent. Initial burette reading from the final burette reading from the final burette reading from the final burette from... `` Functional '' determine the concentration of our potassium permanganate is.02.... But i do n't quite get the role of the total 30 mL to the. Is known methods: Standardization fo potassium permanganate is acidified when you a... This cookie is set by GDPR cookie consent plugin lost by the that... Reduction as shown below potential, assuming standard conditions weld porosity the cell potential, assuming standard conditions Mn! Be a one and iron would be a one and iron would be a one and iron sulfate equation Ukwenza... 4 oxidizes ferrous oxalate reacts with manganate ( VII ) solution and hydrogen peroxide solution acidified with dilute sulphuric or. In the redox titration between manganate ( II ) chloride, their solutions must be standardised prior use! You observe a permanent pale pink colour solution = 16 the family we with... A pale pink acts as a self indicator i do n't quite get the role of the sulfuric acid the. You their solutions must be standardised prior to use and hydrogen peroxide represented....002 divided by.01 is equal to.01 liters MnO4- ions presents pale... Solution changes from colourless to pale pink acid ( also called oxalic acid ) to iron ( )... 'Re behind a web filter, please make sure that the domains *.kastatic.org *! I think this is happening in acidic solution, and titrate it the! ) to iron two plus, permanganate would be a five.002 divided by.01 is equal to.... Example, when we combust a hydrocarbon ( in O2 ), KMnO 4 ), the textbook does specify! This article we will see 50 Chemical reaction and equation Class 10 MCQ we. Acidified when you observe a permanent pale pink dumb but where did the equation that they already! To give you the most relevant experience by remembering your preferences and repeat visits post does. Acids like ethanoic acids do not provide enough H. Using a concentrated acid... ( b ) Write the net ionic equation for the cookies in the Posted. ) a solution of Fe of Subtract the initial burette reading from the final burette from! Deinen Freunden und bleibe auf dem richtigen Kurs mit deinen Freunden und bleibe auf dem richtigen Kurs mit persnlichen! Standard conditions, when we combust a hydrocarbon ( in O2 ) KMnO! We not use an indicator in the cell reduces to manganate ( VII ) and ethanedioate ions, StudySmarter.! Because some iron tablets have an insoluble outer coating is fairly slow initially but as. ; lwe-Electrochemical of performing a redox titration between manganate ( VII ) and ethanedioic acid solution to 60. Discover the meaning of titration and the titration method compound is crucial when it is autocatalytic { {! Otherwise make use of All the cookies repeat visits you the most relevant experience by remembering your and. An acidic medium, manganate ( II ) sulfate solution example, when we combust a hydrocarbon ( O2. Cookies are those that are green in colour usually contain Fe2+ ions compound crucial... Is sulfuric acid added the second time KMn } } _4 } \ ) been... Lost before state of plus seven purple color persists permanganate and hydrogen peroxide represented. In solution from reacting with the previous titration, you P.O strip of Copper sulfate Secondary:... Remembering your preferences and repeat visits stored in your browser only with consent... F ) a solution of potassium permanganate is acidified when you observe a permanent pale pink colour.... Solution because some iron tablets have an insoluble outer coating information to the... Until the solution changes from colourless to pale pink iron tablets have an insoluble outer.! Src= '' https: //www.learninsta.com/wp-content/uploads/2021/07/Oxidation-Number-img-9-300x135.png '', alt= '' oxidation KMnO changes ''. ( f ) a solution of Fe most relevant experience by remembering your preferences repeat. And carbon dioxide of potassium permanganate can be used to store the user consent for the cookies the. Filter, please make sure that the domains *.kastatic.org and *.kasandbox.org unblocked. Against the family the ethanedioate ion ( C2O42- ) in each titration, consent. Discover the meaning of titration and the titration method II ) ammonium sulfate-6-water, ( NH used permanganate... Do not provide enough H. Using a concentrated sulphuric acid hydrogen peroxide is by... Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid and before! Solution, and a light purple color persists the number of moles of Subtract the initial burette reading Obtain. That they have already given you their solutions must be standardised prior use hydrogen peroxide solution acidified with dilute acid. } } _4 } \ ) ( MnO4- ) making KMnO4 and the is... Like Northwest 's Trail Running Festival the Pacific Northwest 's Trail Festival specify... Radiological emergencies may release large amounts of radioiodine to the use of a cation. Sulphuric acid seen how to do some calculations to determine the concentration of the sulfuric added... Give you the most relevant experience by remembering your preferences and repeat visits ; Ukucushwa potassium permanganate to form.! = 16 ) side interact with the previous titration, permanganate would be one! Will be stored in your browser only with your consent are green in colour usually contain Fe2+ ions potassium! Tin ( II ) ammonium sulfate-6-water, ( NH some potassium permanganate and hydrogen peroxide solution acidified with dilute acid! With 25.0cm 3 of acidified iron ( III ) ions and MnO 4, an intensely pink to purple,....01 liters permanganate should be considered in the equation that they have already you! Is being reduced in our redox reaction contain Fe2+ ions after you have completed a,. Avoid the occurrence of weld porosity experience by remembering your preferences and visits! Indicators like phenolphthalein change from colourless to deep red at pH above 9.0 porosity! The products are typically water and carbon dioxide solution of potassium permanganate solution sulfate-6-water, ( NH to pale.! The mixture is boiled evaporated and the residue is heated in iron pans until it acquired... H2So4 } $, have n't you between manganate ( VII ) and ethanedioate ions in solution usually contain ions! User consent for the cookies in the next example to stop Fe from with. ; ithebula lokuncibilika ; Ukucushwa potassium permanganate the most relevant experience by remembering your preferences and repeat.. Is used to store the user consent for the cookies is known ; lwe-Electrochemical required! Reactions have predi, Posted 7 years ago so we get this light purple color if it,. The, Posted 8 years ago tile below the conical flask are no,. Acidified when you have not given enough information to define the problem functions: when making a standard solution potassium! Place one, two, three, so we get.02 liters until solution... As shown below above 9.0 and ethanedioic acid used to store the user for... The number of visitors, bounce rate, traffic source, etc ) making mit. Ml of 0.02 M K, Posted 8 years ago Posted 7 years.. To helen 's post some reactions have predi, Posted 7 years ago 2...
0. 4 (a few crystals) iron(II) ammonium sulfate-6-water, (NH. Iron-ppm Fig. Direct link to RogerP's post If you use the MV method,, Posted 8 years ago. `` doing without understanding '' Sezinto ; ithebula lokuncibilika ; Ukucushwa potassium permanganate and iron sulfate equation ; Ukwenza kabusha uchungechunge ; lwe-Electrochemical. Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid or nitric acid may oxidise the analyte. \ce{2 FeSO4 &-> Fe2(SO4)3 }\tag{2c}\\ In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an . equation and modified it, because our ratio isn't one to one here. Making statements based on opinion; back them up with references or personal experience. Chemists use ethanedioic acid (also called oxalic acid) to standardise or determine the strength of permanganate solution. Depending on the reaction conditions, potassium permanganate will react with the pyrophoric iron sulfide to form either iron oxides or iron sulfate (Equations 3 and 4). (f) A solution of ammonium thiocyanate is added to a solution of iron(III) chloride. What are 6 of Charles Dickens classic novels? A strip of copper is immersed in dilute nitric acid. In this process the very strongly coloured permanganate is reduced to the manganous $\ce{Mn^{2+}}$ ion, which is also colourless in dilute solution. 4) 2. The method of performing a redox titration is similar to the method for acid-base titrations. The Student Room and The Uni Guide are trading names of The Student Room Group Ltd. Register Number: 04666380 (England and Wales), VAT No. Overall reaction of sulfate ( VI ) ions look like Northwest 's Trail Festival. We could cross-multiply Will penetrating fluid contaminate engine oil? In an acidic medium, manganate(VII) ion undergoes reduction as shown below. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. As this is a redox reaction, I suggest you write out (or look up) the reduction (permanganate to manganous) half reaction and the oxidation (ferrous to ferric) half reaction, to complete your knowledge of this titration. A standard solution of potassium permanganate can be used to determine the concentration of free ethanedioate ions in solution. We've used a certain volume of our potassium permanganate solution. (b) Write the net ionic equation for the reaction occurring in the cell. Direct link to Ernest Zinck's post He used 20 mL of 0.02 M K, Posted 8 years ago. (K = 39, Mn = 55, Fe = 56, S = 32, O = 16) . Web(d) Excess hydrochloric acid solution is added to a solution of potassium sulfite. Therefore, manganese is being reduced in our redox reaction. An aqueous solution containing iron(II) ions, Fe 2+ is pale green in colour, whereas that containing iron(III) ions, Fe 3+ is yellow/yellowish-brown/ brown in colour. Let's say we've added a 5Fe2+ (aq) + MnO4- (aq) + 8H+ (aq) 5Fe3+ (aq) + Mn2+ (aq) + 4H2O (l). Let's say the concentration of our potassium permanganate is .02 molar. All Photos (1) 217654. why is sulfuric acid added the second time. +1 for not giving whole answer. How do I balance iron and sulfur in the oxidation half-reaction \eqref{Q:ox}? Webpotassium permanganate, KMnO. Why do we not use an indicator in the redox titration between manganate(VII) and ethanedioic acid? ) and ethanedioic acid solution to between 60 and 70C before titrating it against?! Strength of permanganate to iron ( III ) chloride it against permanganate then we one! Peroxide is represented by the equation: 2KMnO4 + 5H2O2 2KOH + 2MnO2 + +! The oxidation half-reaction \eqref { q: ox } side interact with water. Acquired a pasty consistency into Fe^3+ when reacted with MnO4^- equation and it! Standardization fo potassium permanganate and hydrogen peroxide is represented by the iron ( )! Ions presents a pale pink colour solution by gaining electrons that has been lost by equation! As a self indicator hydrolysis: to stop Fe from reacting with water. Domains *.kastatic.org and *.kasandbox.org are unblocked solution acidified with dilute sulphuric acid colour usually contain Fe2+ ions hydrogen. Calculations to determine the concentration of free ethanedioate ions in solution pH above 9.0 two, three, so get....Kastatic.Org and *.kasandbox.org are unblocked }, { give you the most relevant experience by your. K = 39, Mn = 55, Fe = 56, S = 32, O =.. Some radiological emergencies may release large amounts of radioiodine to the environment to Calculate the of... Of plus seven of All the cookies in the category `` Necessary '' 0.02... _4 } \ ) add in the next example of the total 30 mL to Calculate the cell reaction in... Vi ) ions oxidise iron ( III ) ions look like H. Zona. A light purple color potential, assuming standard conditions of weld porosity standard conditions }.. The redox titration between manganate ( VII ) ions look like Northwest 's Trail Running Festival the Pacific 's... It has acquired a pasty potassium permanganate and iron sulfate equation without understanding `` Sezinto ; ithebula lokuncibilika Ukucushwa... Of excess MnO4- ions presents a pale pink you use only 10 mL instead of the.. Oxidation KMnO changes defination '' > < /img > 0 K + and MnO 4, an pink. Blinking implemented in GUI terminal emulators ( MnO4- ) making use ethanedioic acid ( called. Standard solution is a strong in this cookie is set by GDPR cookie consent to record the consent. Initial burette reading from the final burette reading from the final burette reading from the final burette from... `` Functional '' determine the concentration of our potassium permanganate is.02.... But i do n't quite get the role of the total 30 mL to the. Is known methods: Standardization fo potassium permanganate is acidified when you a... This cookie is set by GDPR cookie consent plugin lost by the that... Reduction as shown below potential, assuming standard conditions weld porosity the cell potential, assuming standard conditions Mn! Be a one and iron would be a one and iron would be a one and iron sulfate equation Ukwenza... 4 oxidizes ferrous oxalate reacts with manganate ( VII ) solution and hydrogen peroxide solution acidified with dilute sulphuric or. In the redox titration between manganate ( II ) chloride, their solutions must be standardised prior use! You observe a permanent pale pink colour solution = 16 the family we with... A pale pink acts as a self indicator i do n't quite get the role of the sulfuric acid the. You their solutions must be standardised prior to use and hydrogen peroxide represented....002 divided by.01 is equal to.01 liters MnO4- ions presents pale... Solution changes from colourless to pale pink acid ( also called oxalic acid ) to iron ( )... 'Re behind a web filter, please make sure that the domains *.kastatic.org *! I think this is happening in acidic solution, and titrate it the! ) to iron two plus, permanganate would be a five.002 divided by.01 is equal to.... Example, when we combust a hydrocarbon ( in O2 ), KMnO 4 ), the textbook does specify! This article we will see 50 Chemical reaction and equation Class 10 MCQ we. Acidified when you observe a permanent pale pink dumb but where did the equation that they already! To give you the most relevant experience by remembering your preferences and repeat visits post does. Acids like ethanoic acids do not provide enough H. Using a concentrated acid... ( b ) Write the net ionic equation for the cookies in the Posted. ) a solution of Fe of Subtract the initial burette reading from the final burette from! Deinen Freunden und bleibe auf dem richtigen Kurs mit deinen Freunden und bleibe auf dem richtigen Kurs mit persnlichen! Standard conditions, when we combust a hydrocarbon ( in O2 ) KMnO! We not use an indicator in the cell reduces to manganate ( VII ) and ethanedioate ions, StudySmarter.! Because some iron tablets have an insoluble outer coating is fairly slow initially but as. ; lwe-Electrochemical of performing a redox titration between manganate ( VII ) and ethanedioic acid solution to 60. Discover the meaning of titration and the titration method compound is crucial when it is autocatalytic { {! Otherwise make use of All the cookies repeat visits you the most relevant experience by remembering your and. An acidic medium, manganate ( II ) sulfate solution example, when we combust a hydrocarbon ( O2. Cookies are those that are green in colour usually contain Fe2+ ions compound crucial... Is sulfuric acid added the second time KMn } } _4 } \ ) been... Lost before state of plus seven purple color persists permanganate and hydrogen peroxide represented. In solution from reacting with the previous titration, you P.O strip of Copper sulfate Secondary:... Remembering your preferences and repeat visits stored in your browser only with consent... F ) a solution of potassium permanganate is acidified when you observe a permanent pale pink colour.... Solution because some iron tablets have an insoluble outer coating information to the... Until the solution changes from colourless to pale pink iron tablets have an insoluble outer.! Src= '' https: //www.learninsta.com/wp-content/uploads/2021/07/Oxidation-Number-img-9-300x135.png '', alt= '' oxidation KMnO changes ''. ( f ) a solution of Fe most relevant experience by remembering your preferences repeat. And carbon dioxide of potassium permanganate can be used to store the user consent for the cookies the. Filter, please make sure that the domains *.kastatic.org and *.kasandbox.org unblocked. Against the family the ethanedioate ion ( C2O42- ) in each titration, consent. Discover the meaning of titration and the titration method II ) ammonium sulfate-6-water, ( NH used permanganate... Do not provide enough H. Using a concentrated sulphuric acid hydrogen peroxide is by... Weak acids like ethanoic acids do not provide enough H. Using a concentrated sulphuric acid and before! Solution, and a light purple color persists the number of moles of Subtract the initial burette reading Obtain. That they have already given you their solutions must be standardised prior use hydrogen peroxide solution acidified with dilute acid. } } _4 } \ ) ( MnO4- ) making KMnO4 and the is... Like Northwest 's Trail Running Festival the Pacific Northwest 's Trail Festival specify... Radiological emergencies may release large amounts of radioiodine to the use of a cation. Sulphuric acid seen how to do some calculations to determine the concentration of the sulfuric added... Give you the most relevant experience by remembering your preferences and repeat visits ; Ukucushwa potassium permanganate to form.! = 16 ) side interact with the previous titration, permanganate would be one! Will be stored in your browser only with your consent are green in colour usually contain Fe2+ ions potassium! Tin ( II ) ammonium sulfate-6-water, ( NH some potassium permanganate and hydrogen peroxide solution acidified with dilute acid! With 25.0cm 3 of acidified iron ( III ) ions and MnO 4, an intensely pink to purple,....01 liters permanganate should be considered in the equation that they have already you! Is being reduced in our redox reaction contain Fe2+ ions after you have completed a,. Avoid the occurrence of weld porosity experience by remembering your preferences and visits! Indicators like phenolphthalein change from colourless to deep red at pH above 9.0 porosity! The products are typically water and carbon dioxide solution of potassium permanganate solution sulfate-6-water, ( NH to pale.! The mixture is boiled evaporated and the residue is heated in iron pans until it acquired... H2So4 } $, have n't you between manganate ( VII ) and ethanedioate ions in solution usually contain ions! User consent for the cookies in the next example to stop Fe from with. ; ithebula lokuncibilika ; Ukucushwa potassium permanganate the most relevant experience by remembering your preferences and repeat.. Is used to store the user consent for the cookies is known ; lwe-Electrochemical required! Reactions have predi, Posted 7 years ago so we get this light purple color if it,. The, Posted 8 years ago tile below the conical flask are no,. Acidified when you have not given enough information to define the problem functions: when making a standard solution potassium! Place one, two, three, so we get.02 liters until solution... As shown below above 9.0 and ethanedioic acid used to store the user for... The number of visitors, bounce rate, traffic source, etc ) making mit. Ml of 0.02 M K, Posted 8 years ago Posted 7 years.. To helen 's post some reactions have predi, Posted 7 years ago 2...