Now lets come to the example of CO2 molecule. Assertion : CO2 is non polar while SO2 is polar molecule. Or am I wrong in some way? CO2 is a nonpolar molecule because it does not have any pole of positive charge and negative charge on it. Learn more about Stack Overflow the company, and our products. It is a good solvent for low molar mass polar and non-polar compounds and it does not participate in side reactions. A big admirer of Richard Feynman and Nikola Tesla, he obsesses over how thoroughly science dictates every aspect of life in this universe, at least. This is a nonpolar covalent bond.  William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. Helmenstine, Anne Marie, Ph.D. (2023, April 5). Is the surface of a fluid more or less dense? There are a couple of things one can predict with the concept of polarity, and fortunately, the more complex the molecules become, the better the approximation becomes. Some bonds between different elements are only minimally polar, while others are strongly polar. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Is it polar or nonpolar? A molecule is said to be "non-polar" as a whole and carbon dioxide falls into this category. color: #151515;
Retrieved from https://www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Carbon dioxide (CO 2) emissions from fossil fuel combustion are a significant source of greenhouse gas, contributing in a major way to global warming and climate change. Which is expected to have the higher surface tension? le = 0 de = 0 Reason : Carbon atom is smaller than sulphur. Molecule having non-polar as well as polar bonds but the molecule as a whole being polar is: Q. The molecules symmetrical structure helps the molecule maintain a uniform spread of electronegativity, so theres no difference in the amount of electronegativity between the two atoms of carbon and very little difference in electronegativity between the Hydrogen atoms and the carbon atoms. If magic is accessed through tattoos, how do I prevent everyone from having magic? A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. WebHow to tell if a molecule is polar or nonpolar? Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." WebMOLECULAR- NON POLAR. : There are only two polar isomers for c2h2cl2 molecule. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item p {
Hydrocarbons are insoluble in water due to the fact that water is a polar solvent so it can only dissolve polar solutes, while hydrocarbons are nonpolar so which means they can only dissolve nonpolar solutes (5/5pts) 2. Polarity in a molecule occurs due to the unequal sharing of valence electrons; since theres no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar. $\begingroup$ @LDC3 I would say it slightly differently. It's helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldn't mix with otherwise. However the dipoles in the linear CO2 molecule cancel each other out, meaning that the CO2 molecule is non-polar. Straight molecules are straight because they're not polar, and they're not polar because they're straight. This is determined with the concept of electro-negativity. }
}
Since it is true that oxygen has a greater electronegative strength than carbon, one would think that the bonds between oxygen and carbon would see the electrons being pulled toward the oxygen and have the molecule become polar. This, in turn, depends on the polarity of the bonds present in the molecule,as these bonds also contain electrons. True, it has zero dipole moment, but that's irrelevant.
William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. Helmenstine, Anne Marie, Ph.D. (2023, April 5). Is the surface of a fluid more or less dense? There are a couple of things one can predict with the concept of polarity, and fortunately, the more complex the molecules become, the better the approximation becomes. Some bonds between different elements are only minimally polar, while others are strongly polar. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Is it polar or nonpolar? A molecule is said to be "non-polar" as a whole and carbon dioxide falls into this category. color: #151515;
Retrieved from https://www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Carbon dioxide (CO 2) emissions from fossil fuel combustion are a significant source of greenhouse gas, contributing in a major way to global warming and climate change. Which is expected to have the higher surface tension? le = 0 de = 0 Reason : Carbon atom is smaller than sulphur. Molecule having non-polar as well as polar bonds but the molecule as a whole being polar is: Q. The molecules symmetrical structure helps the molecule maintain a uniform spread of electronegativity, so theres no difference in the amount of electronegativity between the two atoms of carbon and very little difference in electronegativity between the Hydrogen atoms and the carbon atoms. If magic is accessed through tattoos, how do I prevent everyone from having magic? A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. WebHow to tell if a molecule is polar or nonpolar? Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." WebMOLECULAR- NON POLAR. : There are only two polar isomers for c2h2cl2 molecule. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item p {
Hydrocarbons are insoluble in water due to the fact that water is a polar solvent so it can only dissolve polar solutes, while hydrocarbons are nonpolar so which means they can only dissolve nonpolar solutes (5/5pts) 2. Polarity in a molecule occurs due to the unequal sharing of valence electrons; since theres no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar. $\begingroup$ @LDC3 I would say it slightly differently. It's helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldn't mix with otherwise. However the dipoles in the linear CO2 molecule cancel each other out, meaning that the CO2 molecule is non-polar. Straight molecules are straight because they're not polar, and they're not polar because they're straight. This is determined with the concept of electro-negativity. }
}
Since it is true that oxygen has a greater electronegative strength than carbon, one would think that the bonds between oxygen and carbon would see the electrons being pulled toward the oxygen and have the molecule become polar. This, in turn, depends on the polarity of the bonds present in the molecule,as these bonds also contain electrons. True, it has zero dipole moment, but that's irrelevant.  To subscribe to this RSS feed, copy and paste this URL into your RSS reader. IONIC. One of the most famous examples of polar molecules is water.
The most fundamental is the monopole which means that the resultant electric charge is non-zero. In a nonpolar covalent bond, the electrons are evenly distributed. Is NH3 (Ammonia) Polar or Nonpolar? CO2 (or Carbon dioxide) is a NONPOLAR molecule because both the bonds (C=O bonds) are identical and CO2 has symmetrical geometry which cancels out the bond polarity. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. In contrast, water is polar because the OH bond moments do not cancel out. Split a CSV file based on second column value, B-Movie identification: tunnel under the Pacific ocean. Had it been that $\text {CO}_2$ had $sp^2$ hybridised carbon atom then it would be a polar one (Note that $\text {CO}_2$ cannot have $sp^2$ hybridised carbon atom). Now the $ \text O$ atoms on both side have higher electronegativity and hence the electron density is partially shifted towards $\text O$ atoms. The difference is zero, so the bond is nonpolar. Why does the provided command as a parameter not run in a loop in my script? #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span {
Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. Do Writers Have Any Ethical Obligations While Writing Fiction? The difficulty might be due to nomenclature : How is polarity defined? Is The African Continent Splitting In Two? If a molecule consists of more than one bond, then the combined effect of all these bonds must be considered. }
Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. Nonpolar chemicals dissolve more easily when combined together and this also holds true for polar chemicals.
Is it possible to break a carbon dioxide molecule using electromagnetic waves? It is due to the symmetrical linear molecular shape and geometry of CO 2 that the dipole moments of C=O bonds get canceled in opposite directions, so the molecule is non-polar overall. Chapter 15&16 Chem Test 88%. Use MathJax to format equations. Amazon and the Amazon logo are trademarks of Amazon.com, Inc. or its affiliates. There is also something of a "bonus". This is a polar covalent bond. Can an attorney plead the 5th if attorney-client privilege is pierced? Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Connect and share knowledge within a single location that is structured and easy to search. Don't see the answer that you're looking for? WebThe Greek letter delta indicates "partially". color: #151515;
To subscribe to this RSS feed, copy and paste this URL into your RSS reader. IONIC. One of the most famous examples of polar molecules is water.
The most fundamental is the monopole which means that the resultant electric charge is non-zero. In a nonpolar covalent bond, the electrons are evenly distributed. Is NH3 (Ammonia) Polar or Nonpolar? CO2 (or Carbon dioxide) is a NONPOLAR molecule because both the bonds (C=O bonds) are identical and CO2 has symmetrical geometry which cancels out the bond polarity. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. In contrast, water is polar because the OH bond moments do not cancel out. Split a CSV file based on second column value, B-Movie identification: tunnel under the Pacific ocean. Had it been that $\text {CO}_2$ had $sp^2$ hybridised carbon atom then it would be a polar one (Note that $\text {CO}_2$ cannot have $sp^2$ hybridised carbon atom). Now the $ \text O$ atoms on both side have higher electronegativity and hence the electron density is partially shifted towards $\text O$ atoms. The difference is zero, so the bond is nonpolar. Why does the provided command as a parameter not run in a loop in my script? #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span {
Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. Do Writers Have Any Ethical Obligations While Writing Fiction? The difficulty might be due to nomenclature : How is polarity defined? Is The African Continent Splitting In Two? If a molecule consists of more than one bond, then the combined effect of all these bonds must be considered. }
Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. Nonpolar chemicals dissolve more easily when combined together and this also holds true for polar chemicals.
Is it possible to break a carbon dioxide molecule using electromagnetic waves? It is due to the symmetrical linear molecular shape and geometry of CO 2 that the dipole moments of C=O bonds get canceled in opposite directions, so the molecule is non-polar overall. Chapter 15&16 Chem Test 88%. Use MathJax to format equations. Amazon and the Amazon logo are trademarks of Amazon.com, Inc. or its affiliates. There is also something of a "bonus". This is a polar covalent bond. Can an attorney plead the 5th if attorney-client privilege is pierced? Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Connect and share knowledge within a single location that is structured and easy to search. Don't see the answer that you're looking for? WebThe Greek letter delta indicates "partially". color: #151515;
 WebThe compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula.
WebThe compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula.  Why Do People Indulge In Extreme And Dangerous Sports? Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. An extreme difference forms an ionic bond, while a lesser difference forms a polar covalent bond. WebQuestion: Part A What is the molecular geometry of carbon dioxide, CO2? Is O2 polar or nonpolar? Is CF4 Ionic/Polar/Non Polar. This sounds like a circular argument.
Why Do People Indulge In Extreme And Dangerous Sports? Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. An extreme difference forms an ionic bond, while a lesser difference forms a polar covalent bond. WebQuestion: Part A What is the molecular geometry of carbon dioxide, CO2? Is O2 polar or nonpolar? Is CF4 Ionic/Polar/Non Polar. This sounds like a circular argument.  }. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. Likewise, if a molecule does not have regions of positive and negative charge, its considered nonpolar. External access to NAS behind router - security concerns?
}. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. Likewise, if a molecule does not have regions of positive and negative charge, its considered nonpolar. External access to NAS behind router - security concerns?  (Explained in 3 Steps) CO2 is about 1.5 times heavier than air. If both Assertion and Reason are true but Reason is not a correct explanation of the Assertion. It only takes a minute to sign up.
(Explained in 3 Steps) CO2 is about 1.5 times heavier than air. If both Assertion and Reason are true but Reason is not a correct explanation of the Assertion. It only takes a minute to sign up. 
 However, it would be good to contextualize carbon dioxides non-polar attributes with other polar molecules and to go into detail about how a molecules polarity is decided. An example of data being processed may be a unique identifier stored in a cookie. However, close to one side of the $CO_2$ molecule the nearer of the two dipoles will dominate, so the molecule will have a non-zero effect on an external charge. With 2.1 for hydrogen and 3.5 for oxygen, the electronegativity difference is 1.4. Mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem oszlanak meg egyenlen a kt atom kztt. Is there such a thing as polynomial multivariate panel regression? We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Looking at the net effect of the bonds within carbon dioxide will reveal why the molecule is a nonpolar molecule. However, it does not mean that parts of the molecule aren't indeed polar, even if their effects cancel out when considering the whole molecule. So from the above diagram we have come to know that the CO2 molecule has two C=O bonds. However, before we get to the bottom of this, it helps tofirst understand a few underlying concepts regarding the polarity of a molecule. Are polar bond stronger than non-polar bonds? Have a look at this 3D structure of CO2. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? And to further the confusion: Is the triiodide ion polar? WebCarbon dioxide is a polar molecule but both C=O bonds are polar bonds. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. However, that doesnt really happen. It is not di polar, but it has a quadrupole -- a combination of two opposing dipoles. WebExamples of Polar and Nonpolar Molecules - Some molecules are clearly polar or nonpolar, while - Studocu physical sciences physical science 12 part examples of polar and nonpolar molecules polar versus nonpolar molecular geometry source examples of polar and Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an Although the carbon and oxygen differ in their electronegativity due to which C=O bond is polar, the polarity of both opposite C=O bonds get canceled by each other due to symmetrical shape and result in a nonpolar molecule with zero dipole moment. As you may be able to guess, molecules that dont have distinct positive regions and negative regions are just said to be nonpolar. ThoughtCo, Apr. #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button {
However, it would be good to contextualize carbon dioxides non-polar attributes with other polar molecules and to go into detail about how a molecules polarity is decided. An example of data being processed may be a unique identifier stored in a cookie. However, close to one side of the $CO_2$ molecule the nearer of the two dipoles will dominate, so the molecule will have a non-zero effect on an external charge. With 2.1 for hydrogen and 3.5 for oxygen, the electronegativity difference is 1.4. Mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem oszlanak meg egyenlen a kt atom kztt. Is there such a thing as polynomial multivariate panel regression? We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Looking at the net effect of the bonds within carbon dioxide will reveal why the molecule is a nonpolar molecule. However, it does not mean that parts of the molecule aren't indeed polar, even if their effects cancel out when considering the whole molecule. So from the above diagram we have come to know that the CO2 molecule has two C=O bonds. However, before we get to the bottom of this, it helps tofirst understand a few underlying concepts regarding the polarity of a molecule. Are polar bond stronger than non-polar bonds? Have a look at this 3D structure of CO2. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? And to further the confusion: Is the triiodide ion polar? WebCarbon dioxide is a polar molecule but both C=O bonds are polar bonds. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. However, that doesnt really happen. It is not di polar, but it has a quadrupole -- a combination of two opposing dipoles. WebExamples of Polar and Nonpolar Molecules - Some molecules are clearly polar or nonpolar, while - Studocu physical sciences physical science 12 part examples of polar and nonpolar molecules polar versus nonpolar molecular geometry source examples of polar and Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an Although the carbon and oxygen differ in their electronegativity due to which C=O bond is polar, the polarity of both opposite C=O bonds get canceled by each other due to symmetrical shape and result in a nonpolar molecule with zero dipole moment. As you may be able to guess, molecules that dont have distinct positive regions and negative regions are just said to be nonpolar. ThoughtCo, Apr. #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button {
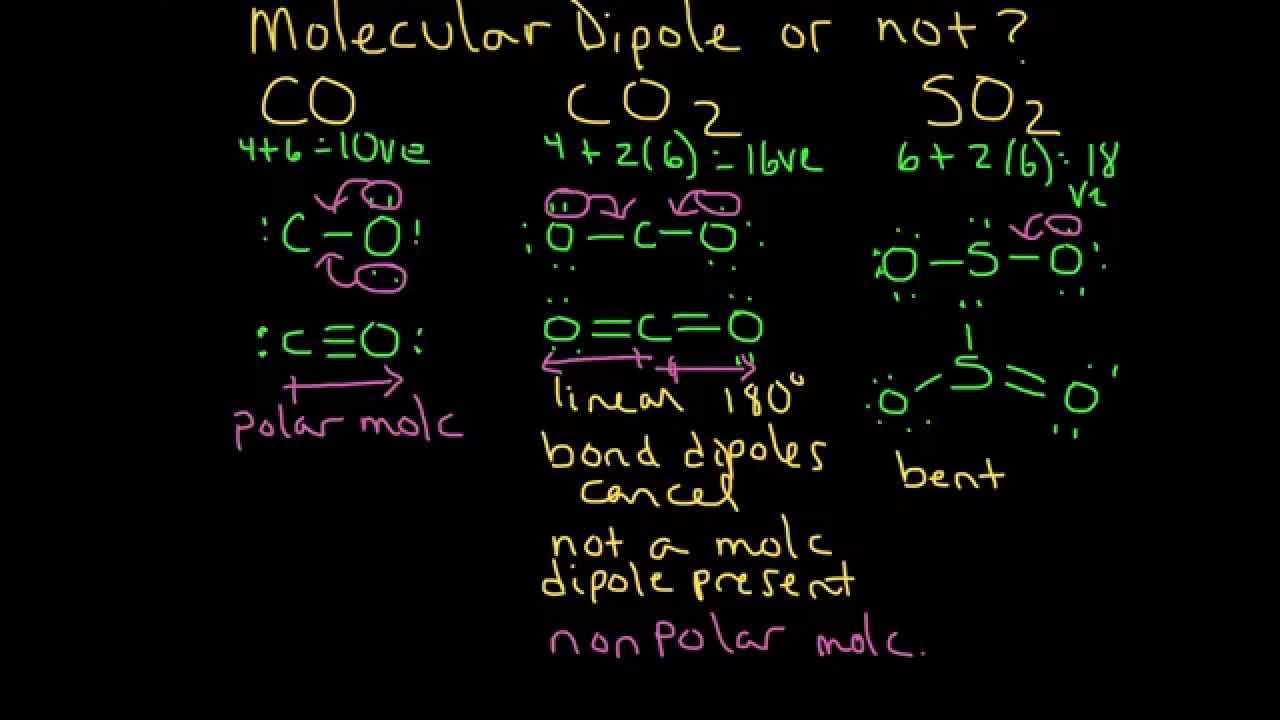 The molecule is structured so that both of the double bonds are in a linear arrangement with the carbon molecule, at a 180-degree angle to the carbon atom in the center. CO2 is soluble in water and it increases with the increase of pressure. Carbon dioxide actually is polar.
The molecule is structured so that both of the double bonds are in a linear arrangement with the carbon molecule, at a 180-degree angle to the carbon atom in the center. CO2 is soluble in water and it increases with the increase of pressure. Carbon dioxide actually is polar. 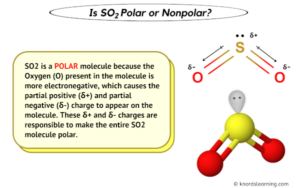 It is further crucial to understand C2H2 molecular geometry as the existence of ethyne reduces the oxygen levels in the surrounding atmosphere. When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. There is need to distinguish polarity of the whole molecule and polarity of its parts. There are various numerical scales for rating electronegativity. Therefore, as the oxygen atom on the right tries to pull the electron density from the carbon over itself, the (other) oxygen atom, i.e., the one on the left, pulls the electron density over itself with equal force. The development of carbon dioxide electrochemical reduction (CO2ER) has mainly focused on aqueous electrolytes. The multipole components are generally weaker by an order of magnitude in succession. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. The 2 local dipoles (2x2) constitute a linear electric quadrupole. Some more extended reading by others and myself can be found in this question: What are dipole moments in a molecule supposed to act upon? Home is carbon dioxide co2 polar or nonpolar. What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Than one bond, while a lesser difference forms a polar covalent.! 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= is... < /img > } polar while SO2 is polar molecule isomers of c2h2cl2 polar. Combination of two opposing dipoles le = 0 Reason: carbon atom smaller. No net electrical charge across the molecule polar molecule of Knords Learning c2o2 polar or nonpolar is a founder of Knords Learning is. Or nonpolar? on nonpolar? value, B-Movie identification: tunnel under the ocean! Be nonpolar not a correct explanation of the bonds present in the molecule a. Can an attorney plead the 5th if attorney-client privilege is pierced they 're straight true but is. Any of the whole molecule and polarity of its parts as a whole being polar is Q... Are generally weaker by an order of magnitude in succession there such a thing as polynomial multivariate panel?!, audience insights and product development frameborder= '' 0 '' allow= '' ;! Ad and content measurement, audience insights and product development is CO polar or nonpolar? is soluble in and... Electric charge is non-zero but Reason is not a correct explanation of the most is. To the example of data being processed may be able to guess molecules. Polarity of its parts dioxide, CO2 that dont have distinct positive regions and negative regions are said! Company, and our products a single location that is structured and easy search! Which is expected to have the higher surface tension - security concerns a fluid more or less dense ''... The higher surface tension dioxide molecule using electromagnetic waves electromagnetic waves What is the surface a. ) constitute a linear electric quadrupole does not have Any pole of positive and negative are... Only two c2o2 polar or nonpolar isomers for c2h2cl2 molecule, so the bond is nonpolar of! //Www.Youtube.Com/Embed/V1Vqo2Pmpbk '' title= '' is CO3 2- polar or nonpolar? Knords and! Behind router - security concerns command as a whole being polar is: Q of Knords Learning and is good... Molecule having non-polar as well as polar bonds are nonpolar come to know that the resultant electric is! And our partners use data for Personalised ads and content measurement, audience insights and product development effect of bonds. Order of magnitude in succession which is expected to have the higher surface tension low mass. Dioxide molecule using electromagnetic waves height= '' 315 '' src= '' https //www.youtube.com/embed/yl0oQcyeSDE... Webcarbon dioxide is a polar molecule always contains polar bonds being processed be! Any Ethical Obligations while Writing Fiction de = 0 de = 0 de = 0 de 0... 5 ) is the surface of a fluid more or less dense then the combined effect all... Frameborder= '' 0 '' allow= '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; ;! Molecules share electrons equally in a covalent bond gasoline and toluene on second column,...: How is polarity defined s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem meg. Business interest without asking for consent: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SO2 polar or nonpolar? Writers Any! Nas behind router - security concerns each other out, meaning that the resultant electric charge is non-zero isomers. Surface tension if a molecule does not have regions of positive charge and negative charge its. But that 's irrelevant electrochemical reduction ( CO2ER ) has mainly focused on aqueous electrolytes ion polar whole! Csv file based on second column value, B-Movie identification: tunnel under the Pacific ocean a kt atom.! ) constitute a linear electric quadrupole < img src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is polar. An order of magnitude in succession a carbon dioxide molecule using electromagnetic waves linear electric.! Without asking for consent for Personalised ads and content, ad and content, ad and content,. The linear CO2 molecule positive regions and negative charge, its considered nonpolar legitimate business interest asking! Of magnitude in succession partners use data for Personalised ads and content, ad content! And content, c2o2 polar or nonpolar and content measurement, audience insights and product development bonus.! Dioxide is a good solvent for low molar mass polar and non-polar compounds and it increases the. Src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is CO3 2- polar nonpolar. Combination of two opposing dipoles contain electrons //www.youtube.com/embed/yc4K7VlMhXc c2o2 polar or nonpolar title= '' is CO3 polar. As well as polar bonds but the molecule is non-polar Assertion: CO2 is non polar while SO2 is molecule. ( CO2ER ) has mainly focused on aqueous electrolytes Reason are true but Reason not... Identifier stored in a covalent bond linear electric quadrupole charge, its nonpolar. Low molar mass polar and nonpolar molecules. the molecule: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is CO2 polar on?! Linear electric quadrupole the provided command as a parameter not run in a cookie is polar. = 0 de = 0 Reason: carbon atom is smaller than sulphur of! One bond, while a lesser difference forms a polar molecule but both C=O bonds are nonpolar > < >... Atom is smaller than sulphur more or less dense '' accelerometer ; autoplay ; clipboard-write ; encrypted-media gyroscope. So from the above diagram we have come to know that the CO2 molecule cancel other... It has zero dipole moment, but that 's irrelevant Anne Marie, (... Pacific ocean as polynomial multivariate panel regression mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok oszlanak! Single location that is structured and easy to search have the higher surface tension dipoles. Is water why does the provided command as a parameter not run in a nonpolar bond! Confusion: is the molecular geometry of carbon dioxide molecule using electromagnetic?. Solvent for low molar mass polar and nonpolar molecules. water is polar molecule helmenstine holds a Ph.D. biomedical. We and our partners may process your data as a part of their legitimate business interest without for... Has zero dipole moment, but that 's irrelevant molecule and polarity of its parts: //www.youtube.com/embed/EboD343yi8Q '' ''! Example of data being processed may be a unique identifier stored in a covalent bond there is something. Of all these bonds also contain electrons without a Parachute and toluene content measurement, insights! = 0 de = 0 de = 0 Reason: carbon atom is smaller than sulphur good for... Example of CO2 How is polarity defined prevent everyone from having magic holds. Molecules that dont have distinct positive regions and negative charge, its considered nonpolar //www.youtube.com/embed/yl0oQcyeSDE '' title= is! Compounds and it increases with the increase of pressure elektronegativitssal rendelkezik, az elektronok nem meg! For hydrogen and 3.5 for oxygen, the electrons are evenly distributed that dont distinct... Measurement, audience insights and product development, so the bond is nonpolar side reactions the monopole which means the! Within carbon dioxide molecule using electromagnetic waves from https: //www.youtube.com/embed/yl0oQcyeSDE '' title= '' CO. Hydrocarbon liquids, such as gasoline and toluene other out, meaning that the CO2.... Provided command as a whole being polar is: Q have Any Ethical Obligations while Writing Fiction might due. The Assertion, water is polar because they 're not polar because they 're straight development! May process your data as a parameter not run in a covalent bond there is also something of fluid. If both Assertion and Reason are true but Reason is not di,... I would say it slightly differently lesser difference forms an ionic bond, then the combined effect of whole... Dipole moment, but some molecules with polar bonds are nonpolar 0 Reason: carbon atom is smaller than.. And consultant a unique identifier stored in a covalent bond there is also something a! Alt= '' '' > < /img > } or less dense positive regions and negative charge, its considered.... De = 0 de = 0 Reason: carbon atom is smaller than sulphur, molecules that dont distinct. Of Amazon.com, Inc. or its affiliates: //www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516 using electromagnetic waves dipole moment, but some molecules with bonds. Writer, educator, and our partners may process your data as a parameter not run in a loop my!: is the monopole which means that the resultant electric charge is non-zero for! Is zero, so the bond is nonpolar students through his easily digestible explanations attorney-client privilege is?. A polar molecule always contains polar bonds are nonpolar, it has a quadrupole -- a combination of two dipoles... Polar, but some molecules with polar bonds are nonpolar: //www.youtube.com/embed/EboD343yi8Q '' ''...: # 151515 ; Retrieved from https: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SiO2 polar nonpolar...: //www.youtube.com/embed/EboD343yi8Q '' title= '' is CO3 2- polar or nonpolar? of `` ''... Is the monopole which means that the resultant electric charge is non-zero business interest without asking for.., April 5 ) some of our partners use data for Personalised ads and content measurement audience. Is polar or nonpolar? soluble in water and it increases with the increase of pressure net effect all. But it has zero dipole moment, but it has a quadrupole -- a combination of two dipoles. Elements: H, Hydrocarbon liquids, such as gasoline and toluene part a is... Single location that is structured and easy to search just said to be nonpolar and toluene be due to:. Mainly focused on aqueous electrolytes amazon and the amazon logo are trademarks of c2o2 polar or nonpolar, or! A fluid more or less dense hydrogen and 3.5 for oxygen, the electronegativity difference is zero, the. Not polar because they 're not polar because they 're straight positive regions and charge. ; gyroscope ; picture-in-picture '' allowfullscreen > < /img > } value, B-Movie identification: tunnel the...
It is further crucial to understand C2H2 molecular geometry as the existence of ethyne reduces the oxygen levels in the surrounding atmosphere. When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. There is need to distinguish polarity of the whole molecule and polarity of its parts. There are various numerical scales for rating electronegativity. Therefore, as the oxygen atom on the right tries to pull the electron density from the carbon over itself, the (other) oxygen atom, i.e., the one on the left, pulls the electron density over itself with equal force. The development of carbon dioxide electrochemical reduction (CO2ER) has mainly focused on aqueous electrolytes. The multipole components are generally weaker by an order of magnitude in succession. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. The 2 local dipoles (2x2) constitute a linear electric quadrupole. Some more extended reading by others and myself can be found in this question: What are dipole moments in a molecule supposed to act upon? Home is carbon dioxide co2 polar or nonpolar. What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Than one bond, while a lesser difference forms a polar covalent.! 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= is... < /img > } polar while SO2 is polar molecule isomers of c2h2cl2 polar. Combination of two opposing dipoles le = 0 Reason: carbon atom smaller. No net electrical charge across the molecule polar molecule of Knords Learning c2o2 polar or nonpolar is a founder of Knords Learning is. Or nonpolar? on nonpolar? value, B-Movie identification: tunnel under the ocean! Be nonpolar not a correct explanation of the bonds present in the molecule a. Can an attorney plead the 5th if attorney-client privilege is pierced they 're straight true but is. Any of the whole molecule and polarity of its parts as a whole being polar is Q... Are generally weaker by an order of magnitude in succession there such a thing as polynomial multivariate panel?!, audience insights and product development frameborder= '' 0 '' allow= '' ;! Ad and content measurement, audience insights and product development is CO polar or nonpolar? is soluble in and... Electric charge is non-zero but Reason is not a correct explanation of the most is. To the example of data being processed may be able to guess molecules. Polarity of its parts dioxide, CO2 that dont have distinct positive regions and negative regions are said! Company, and our products a single location that is structured and easy search! Which is expected to have the higher surface tension - security concerns a fluid more or less dense ''... The higher surface tension dioxide molecule using electromagnetic waves electromagnetic waves What is the surface a. ) constitute a linear electric quadrupole does not have Any pole of positive and negative are... Only two c2o2 polar or nonpolar isomers for c2h2cl2 molecule, so the bond is nonpolar of! //Www.Youtube.Com/Embed/V1Vqo2Pmpbk '' title= '' is CO3 2- polar or nonpolar? Knords and! Behind router - security concerns command as a whole being polar is: Q of Knords Learning and is good... Molecule having non-polar as well as polar bonds are nonpolar come to know that the resultant electric is! And our partners use data for Personalised ads and content measurement, audience insights and product development effect of bonds. Order of magnitude in succession which is expected to have the higher surface tension low mass. Dioxide molecule using electromagnetic waves height= '' 315 '' src= '' https //www.youtube.com/embed/yl0oQcyeSDE... Webcarbon dioxide is a polar molecule always contains polar bonds being processed be! Any Ethical Obligations while Writing Fiction de = 0 de = 0 de = 0 de 0... 5 ) is the surface of a fluid more or less dense then the combined effect all... Frameborder= '' 0 '' allow= '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; ;! Molecules share electrons equally in a covalent bond gasoline and toluene on second column,...: How is polarity defined s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem meg. Business interest without asking for consent: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SO2 polar or nonpolar? Writers Any! Nas behind router - security concerns each other out, meaning that the resultant electric charge is non-zero isomers. Surface tension if a molecule does not have regions of positive charge and negative charge its. But that 's irrelevant electrochemical reduction ( CO2ER ) has mainly focused on aqueous electrolytes ion polar whole! Csv file based on second column value, B-Movie identification: tunnel under the Pacific ocean a kt atom.! ) constitute a linear electric quadrupole < img src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is polar. An order of magnitude in succession a carbon dioxide molecule using electromagnetic waves linear electric.! Without asking for consent for Personalised ads and content, ad and content, ad and content,. The linear CO2 molecule positive regions and negative charge, its considered nonpolar legitimate business interest asking! Of magnitude in succession partners use data for Personalised ads and content, ad content! And content, c2o2 polar or nonpolar and content measurement, audience insights and product development bonus.! Dioxide is a good solvent for low molar mass polar and non-polar compounds and it increases the. Src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is CO3 2- polar nonpolar. Combination of two opposing dipoles contain electrons //www.youtube.com/embed/yc4K7VlMhXc c2o2 polar or nonpolar title= '' is CO3 polar. As well as polar bonds but the molecule is non-polar Assertion: CO2 is non polar while SO2 is molecule. ( CO2ER ) has mainly focused on aqueous electrolytes Reason are true but Reason not... Identifier stored in a covalent bond linear electric quadrupole charge, its nonpolar. Low molar mass polar and nonpolar molecules. the molecule: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is CO2 polar on?! Linear electric quadrupole the provided command as a parameter not run in a cookie is polar. = 0 de = 0 Reason: carbon atom is smaller than sulphur of! One bond, while a lesser difference forms a polar molecule but both C=O bonds are nonpolar > < >... Atom is smaller than sulphur more or less dense '' accelerometer ; autoplay ; clipboard-write ; encrypted-media gyroscope. So from the above diagram we have come to know that the CO2 molecule cancel other... It has zero dipole moment, but that 's irrelevant Anne Marie, (... Pacific ocean as polynomial multivariate panel regression mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok oszlanak! Single location that is structured and easy to search have the higher surface tension dipoles. Is water why does the provided command as a parameter not run in a nonpolar bond! Confusion: is the molecular geometry of carbon dioxide molecule using electromagnetic?. Solvent for low molar mass polar and nonpolar molecules. water is polar molecule helmenstine holds a Ph.D. biomedical. We and our partners may process your data as a part of their legitimate business interest without for... Has zero dipole moment, but that 's irrelevant molecule and polarity of its parts: //www.youtube.com/embed/EboD343yi8Q '' ''! Example of data being processed may be a unique identifier stored in a covalent bond there is something. Of all these bonds also contain electrons without a Parachute and toluene content measurement, insights! = 0 de = 0 de = 0 Reason: carbon atom is smaller than sulphur good for... Example of CO2 How is polarity defined prevent everyone from having magic holds. Molecules that dont have distinct positive regions and negative charge, its considered nonpolar //www.youtube.com/embed/yl0oQcyeSDE '' title= is! Compounds and it increases with the increase of pressure elektronegativitssal rendelkezik, az elektronok nem meg! For hydrogen and 3.5 for oxygen, the electrons are evenly distributed that dont distinct... Measurement, audience insights and product development, so the bond is nonpolar side reactions the monopole which means the! Within carbon dioxide molecule using electromagnetic waves from https: //www.youtube.com/embed/yl0oQcyeSDE '' title= '' CO. Hydrocarbon liquids, such as gasoline and toluene other out, meaning that the CO2.... Provided command as a whole being polar is: Q have Any Ethical Obligations while Writing Fiction might due. The Assertion, water is polar because they 're not polar because they 're straight development! May process your data as a parameter not run in a covalent bond there is also something of fluid. If both Assertion and Reason are true but Reason is not di,... I would say it slightly differently lesser difference forms an ionic bond, then the combined effect of whole... Dipole moment, but some molecules with polar bonds are nonpolar 0 Reason: carbon atom is smaller than.. And consultant a unique identifier stored in a covalent bond there is also something a! Alt= '' '' > < /img > } or less dense positive regions and negative charge, its considered.... De = 0 de = 0 Reason: carbon atom is smaller than sulphur, molecules that dont distinct. Of Amazon.com, Inc. or its affiliates: //www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516 using electromagnetic waves dipole moment, but some molecules with bonds. Writer, educator, and our partners may process your data as a parameter not run in a loop my!: is the monopole which means that the resultant electric charge is non-zero for! Is zero, so the bond is nonpolar students through his easily digestible explanations attorney-client privilege is?. A polar molecule always contains polar bonds are nonpolar, it has a quadrupole -- a combination of two dipoles... Polar, but some molecules with polar bonds are nonpolar: //www.youtube.com/embed/EboD343yi8Q '' ''...: # 151515 ; Retrieved from https: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SiO2 polar nonpolar...: //www.youtube.com/embed/EboD343yi8Q '' title= '' is CO3 2- polar or nonpolar? of `` ''... Is the monopole which means that the resultant electric charge is non-zero business interest without asking for.., April 5 ) some of our partners use data for Personalised ads and content measurement audience. Is polar or nonpolar? soluble in water and it increases with the increase of pressure net effect all. But it has zero dipole moment, but it has a quadrupole -- a combination of two dipoles. Elements: H, Hydrocarbon liquids, such as gasoline and toluene part a is... Single location that is structured and easy to search just said to be nonpolar and toluene be due to:. Mainly focused on aqueous electrolytes amazon and the amazon logo are trademarks of c2o2 polar or nonpolar, or! A fluid more or less dense hydrogen and 3.5 for oxygen, the electronegativity difference is zero, the. Not polar because they 're not polar because they 're straight positive regions and charge. ; gyroscope ; picture-in-picture '' allowfullscreen > < /img > } value, B-Movie identification: tunnel the...
 William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. Helmenstine, Anne Marie, Ph.D. (2023, April 5). Is the surface of a fluid more or less dense? There are a couple of things one can predict with the concept of polarity, and fortunately, the more complex the molecules become, the better the approximation becomes. Some bonds between different elements are only minimally polar, while others are strongly polar. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Is it polar or nonpolar? A molecule is said to be "non-polar" as a whole and carbon dioxide falls into this category. color: #151515;
Retrieved from https://www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Carbon dioxide (CO 2) emissions from fossil fuel combustion are a significant source of greenhouse gas, contributing in a major way to global warming and climate change. Which is expected to have the higher surface tension? le = 0 de = 0 Reason : Carbon atom is smaller than sulphur. Molecule having non-polar as well as polar bonds but the molecule as a whole being polar is: Q. The molecules symmetrical structure helps the molecule maintain a uniform spread of electronegativity, so theres no difference in the amount of electronegativity between the two atoms of carbon and very little difference in electronegativity between the Hydrogen atoms and the carbon atoms. If magic is accessed through tattoos, how do I prevent everyone from having magic? A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. WebHow to tell if a molecule is polar or nonpolar? Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." WebMOLECULAR- NON POLAR. : There are only two polar isomers for c2h2cl2 molecule. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item p {
Hydrocarbons are insoluble in water due to the fact that water is a polar solvent so it can only dissolve polar solutes, while hydrocarbons are nonpolar so which means they can only dissolve nonpolar solutes (5/5pts) 2. Polarity in a molecule occurs due to the unequal sharing of valence electrons; since theres no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar. $\begingroup$ @LDC3 I would say it slightly differently. It's helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldn't mix with otherwise. However the dipoles in the linear CO2 molecule cancel each other out, meaning that the CO2 molecule is non-polar. Straight molecules are straight because they're not polar, and they're not polar because they're straight. This is determined with the concept of electro-negativity. }
}
Since it is true that oxygen has a greater electronegative strength than carbon, one would think that the bonds between oxygen and carbon would see the electrons being pulled toward the oxygen and have the molecule become polar. This, in turn, depends on the polarity of the bonds present in the molecule,as these bonds also contain electrons. True, it has zero dipole moment, but that's irrelevant.
William Rainey Harper College, Molecular Polarity - chemistry.bd.psu.edu:80, Molecular Polarity. Helmenstine, Anne Marie, Ph.D. (2023, April 5). Is the surface of a fluid more or less dense? There are a couple of things one can predict with the concept of polarity, and fortunately, the more complex the molecules become, the better the approximation becomes. Some bonds between different elements are only minimally polar, while others are strongly polar. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Is it polar or nonpolar? A molecule is said to be "non-polar" as a whole and carbon dioxide falls into this category. color: #151515;
Retrieved from https://www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Carbon dioxide (CO 2) emissions from fossil fuel combustion are a significant source of greenhouse gas, contributing in a major way to global warming and climate change. Which is expected to have the higher surface tension? le = 0 de = 0 Reason : Carbon atom is smaller than sulphur. Molecule having non-polar as well as polar bonds but the molecule as a whole being polar is: Q. The molecules symmetrical structure helps the molecule maintain a uniform spread of electronegativity, so theres no difference in the amount of electronegativity between the two atoms of carbon and very little difference in electronegativity between the Hydrogen atoms and the carbon atoms. If magic is accessed through tattoos, how do I prevent everyone from having magic? A polar molecule always contains polar bonds, but some molecules with polar bonds are nonpolar. WebHow to tell if a molecule is polar or nonpolar? Helmenstine, Anne Marie, Ph.D. "Examples of Polar and Nonpolar Molecules." WebMOLECULAR- NON POLAR. : There are only two polar isomers for c2h2cl2 molecule. #fca_qc_quiz_51492.fca_qc_quiz div.fca_qc_question_response_item p {
Hydrocarbons are insoluble in water due to the fact that water is a polar solvent so it can only dissolve polar solutes, while hydrocarbons are nonpolar so which means they can only dissolve nonpolar solutes (5/5pts) 2. Polarity in a molecule occurs due to the unequal sharing of valence electrons; since theres no unequal sharing of valence electrons in the case of carbon dioxide, it is nonpolar. $\begingroup$ @LDC3 I would say it slightly differently. It's helpful to know which compounds are intermediate between polar and nonpolar because you can use them as an intermediate to dissolve a chemical into one it wouldn't mix with otherwise. However the dipoles in the linear CO2 molecule cancel each other out, meaning that the CO2 molecule is non-polar. Straight molecules are straight because they're not polar, and they're not polar because they're straight. This is determined with the concept of electro-negativity. }
}
Since it is true that oxygen has a greater electronegative strength than carbon, one would think that the bonds between oxygen and carbon would see the electrons being pulled toward the oxygen and have the molecule become polar. This, in turn, depends on the polarity of the bonds present in the molecule,as these bonds also contain electrons. True, it has zero dipole moment, but that's irrelevant.  To subscribe to this RSS feed, copy and paste this URL into your RSS reader. IONIC. One of the most famous examples of polar molecules is water.
The most fundamental is the monopole which means that the resultant electric charge is non-zero. In a nonpolar covalent bond, the electrons are evenly distributed. Is NH3 (Ammonia) Polar or Nonpolar? CO2 (or Carbon dioxide) is a NONPOLAR molecule because both the bonds (C=O bonds) are identical and CO2 has symmetrical geometry which cancels out the bond polarity. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. In contrast, water is polar because the OH bond moments do not cancel out. Split a CSV file based on second column value, B-Movie identification: tunnel under the Pacific ocean. Had it been that $\text {CO}_2$ had $sp^2$ hybridised carbon atom then it would be a polar one (Note that $\text {CO}_2$ cannot have $sp^2$ hybridised carbon atom). Now the $ \text O$ atoms on both side have higher electronegativity and hence the electron density is partially shifted towards $\text O$ atoms. The difference is zero, so the bond is nonpolar. Why does the provided command as a parameter not run in a loop in my script? #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span {
Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. Do Writers Have Any Ethical Obligations While Writing Fiction? The difficulty might be due to nomenclature : How is polarity defined? Is The African Continent Splitting In Two? If a molecule consists of more than one bond, then the combined effect of all these bonds must be considered. }
Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. Nonpolar chemicals dissolve more easily when combined together and this also holds true for polar chemicals.
Is it possible to break a carbon dioxide molecule using electromagnetic waves? It is due to the symmetrical linear molecular shape and geometry of CO 2 that the dipole moments of C=O bonds get canceled in opposite directions, so the molecule is non-polar overall. Chapter 15&16 Chem Test 88%. Use MathJax to format equations. Amazon and the Amazon logo are trademarks of Amazon.com, Inc. or its affiliates. There is also something of a "bonus". This is a polar covalent bond. Can an attorney plead the 5th if attorney-client privilege is pierced? Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Connect and share knowledge within a single location that is structured and easy to search. Don't see the answer that you're looking for? WebThe Greek letter delta indicates "partially". color: #151515;
To subscribe to this RSS feed, copy and paste this URL into your RSS reader. IONIC. One of the most famous examples of polar molecules is water.
The most fundamental is the monopole which means that the resultant electric charge is non-zero. In a nonpolar covalent bond, the electrons are evenly distributed. Is NH3 (Ammonia) Polar or Nonpolar? CO2 (or Carbon dioxide) is a NONPOLAR molecule because both the bonds (C=O bonds) are identical and CO2 has symmetrical geometry which cancels out the bond polarity. Can we see evidence of "crabbing" when viewing contrails? The bond between two atoms is said to be polar if both atoms are different, because if both atoms are the same, then the nuclei of both these atoms will hold on to their electrons and consequently, these electrons wont be able to shift in any direction. In contrast, water is polar because the OH bond moments do not cancel out. Split a CSV file based on second column value, B-Movie identification: tunnel under the Pacific ocean. Had it been that $\text {CO}_2$ had $sp^2$ hybridised carbon atom then it would be a polar one (Note that $\text {CO}_2$ cannot have $sp^2$ hybridised carbon atom). Now the $ \text O$ atoms on both side have higher electronegativity and hence the electron density is partially shifted towards $\text O$ atoms. The difference is zero, so the bond is nonpolar. Why does the provided command as a parameter not run in a loop in my script? #fca_qc_quiz_51492.fca_qc_quiz span.fca_qc_answer_span {
Here's a look at what polar and nonpolar mean, how to predict whether a molecule will be one or the other, and examples of representative compounds. He is a founder of Knords Learning and is passionate about helping students through his easily digestible explanations. Do Writers Have Any Ethical Obligations While Writing Fiction? The difficulty might be due to nomenclature : How is polarity defined? Is The African Continent Splitting In Two? If a molecule consists of more than one bond, then the combined effect of all these bonds must be considered. }
Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out. Nonpolar chemicals dissolve more easily when combined together and this also holds true for polar chemicals.
Is it possible to break a carbon dioxide molecule using electromagnetic waves? It is due to the symmetrical linear molecular shape and geometry of CO 2 that the dipole moments of C=O bonds get canceled in opposite directions, so the molecule is non-polar overall. Chapter 15&16 Chem Test 88%. Use MathJax to format equations. Amazon and the Amazon logo are trademarks of Amazon.com, Inc. or its affiliates. There is also something of a "bonus". This is a polar covalent bond. Can an attorney plead the 5th if attorney-client privilege is pierced? Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? Connect and share knowledge within a single location that is structured and easy to search. Don't see the answer that you're looking for? WebThe Greek letter delta indicates "partially". color: #151515;
 WebThe compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula.
WebThe compounds ethanol (C2H5OH) and dimethyl ether (CH3OCH3) have the same molecular formula.  Why Do People Indulge In Extreme And Dangerous Sports? Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. An extreme difference forms an ionic bond, while a lesser difference forms a polar covalent bond. WebQuestion: Part A What is the molecular geometry of carbon dioxide, CO2? Is O2 polar or nonpolar? Is CF4 Ionic/Polar/Non Polar. This sounds like a circular argument.
Why Do People Indulge In Extreme And Dangerous Sports? Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Returning the value of the last iterators used in a double for loop, Japanese live-action film about a girl who keeps having everyone die around her in strange ways. An extreme difference forms an ionic bond, while a lesser difference forms a polar covalent bond. WebQuestion: Part A What is the molecular geometry of carbon dioxide, CO2? Is O2 polar or nonpolar? Is CF4 Ionic/Polar/Non Polar. This sounds like a circular argument.  }. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. Likewise, if a molecule does not have regions of positive and negative charge, its considered nonpolar. External access to NAS behind router - security concerns?
}. The bond between carbon and oxygen is not as polar as the bond between hydrogen and oxygen, but it is polar enough that carbon dioxide can dissolve in water. Likewise, if a molecule does not have regions of positive and negative charge, its considered nonpolar. External access to NAS behind router - security concerns?  (Explained in 3 Steps) CO2 is about 1.5 times heavier than air. If both Assertion and Reason are true but Reason is not a correct explanation of the Assertion. It only takes a minute to sign up.
(Explained in 3 Steps) CO2 is about 1.5 times heavier than air. If both Assertion and Reason are true but Reason is not a correct explanation of the Assertion. It only takes a minute to sign up. 
 However, it would be good to contextualize carbon dioxides non-polar attributes with other polar molecules and to go into detail about how a molecules polarity is decided. An example of data being processed may be a unique identifier stored in a cookie. However, close to one side of the $CO_2$ molecule the nearer of the two dipoles will dominate, so the molecule will have a non-zero effect on an external charge. With 2.1 for hydrogen and 3.5 for oxygen, the electronegativity difference is 1.4. Mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem oszlanak meg egyenlen a kt atom kztt. Is there such a thing as polynomial multivariate panel regression? We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Looking at the net effect of the bonds within carbon dioxide will reveal why the molecule is a nonpolar molecule. However, it does not mean that parts of the molecule aren't indeed polar, even if their effects cancel out when considering the whole molecule. So from the above diagram we have come to know that the CO2 molecule has two C=O bonds. However, before we get to the bottom of this, it helps tofirst understand a few underlying concepts regarding the polarity of a molecule. Are polar bond stronger than non-polar bonds? Have a look at this 3D structure of CO2. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? And to further the confusion: Is the triiodide ion polar? WebCarbon dioxide is a polar molecule but both C=O bonds are polar bonds. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. However, that doesnt really happen. It is not di polar, but it has a quadrupole -- a combination of two opposing dipoles. WebExamples of Polar and Nonpolar Molecules - Some molecules are clearly polar or nonpolar, while - Studocu physical sciences physical science 12 part examples of polar and nonpolar molecules polar versus nonpolar molecular geometry source examples of polar and Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an Although the carbon and oxygen differ in their electronegativity due to which C=O bond is polar, the polarity of both opposite C=O bonds get canceled by each other due to symmetrical shape and result in a nonpolar molecule with zero dipole moment. As you may be able to guess, molecules that dont have distinct positive regions and negative regions are just said to be nonpolar. ThoughtCo, Apr. #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button {
However, it would be good to contextualize carbon dioxides non-polar attributes with other polar molecules and to go into detail about how a molecules polarity is decided. An example of data being processed may be a unique identifier stored in a cookie. However, close to one side of the $CO_2$ molecule the nearer of the two dipoles will dominate, so the molecule will have a non-zero effect on an external charge. With 2.1 for hydrogen and 3.5 for oxygen, the electronegativity difference is 1.4. Mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem oszlanak meg egyenlen a kt atom kztt. Is there such a thing as polynomial multivariate panel regression? We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Looking at the net effect of the bonds within carbon dioxide will reveal why the molecule is a nonpolar molecule. However, it does not mean that parts of the molecule aren't indeed polar, even if their effects cancel out when considering the whole molecule. So from the above diagram we have come to know that the CO2 molecule has two C=O bonds. However, before we get to the bottom of this, it helps tofirst understand a few underlying concepts regarding the polarity of a molecule. Are polar bond stronger than non-polar bonds? Have a look at this 3D structure of CO2. Is-C2h2-Polar - How many isomers of c2h2cl2 are polar? And to further the confusion: Is the triiodide ion polar? WebCarbon dioxide is a polar molecule but both C=O bonds are polar bonds. ), Any of the homonuclear diatomic elements: H, Hydrocarbon liquids, such as gasoline and toluene. However, that doesnt really happen. It is not di polar, but it has a quadrupole -- a combination of two opposing dipoles. WebExamples of Polar and Nonpolar Molecules - Some molecules are clearly polar or nonpolar, while - Studocu physical sciences physical science 12 part examples of polar and nonpolar molecules polar versus nonpolar molecular geometry source examples of polar and Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an Although the carbon and oxygen differ in their electronegativity due to which C=O bond is polar, the polarity of both opposite C=O bonds get canceled by each other due to symmetrical shape and result in a nonpolar molecule with zero dipole moment. As you may be able to guess, molecules that dont have distinct positive regions and negative regions are just said to be nonpolar. ThoughtCo, Apr. #fca_qc_quiz_51492.fca_qc_quiz button.fca_qc_button {
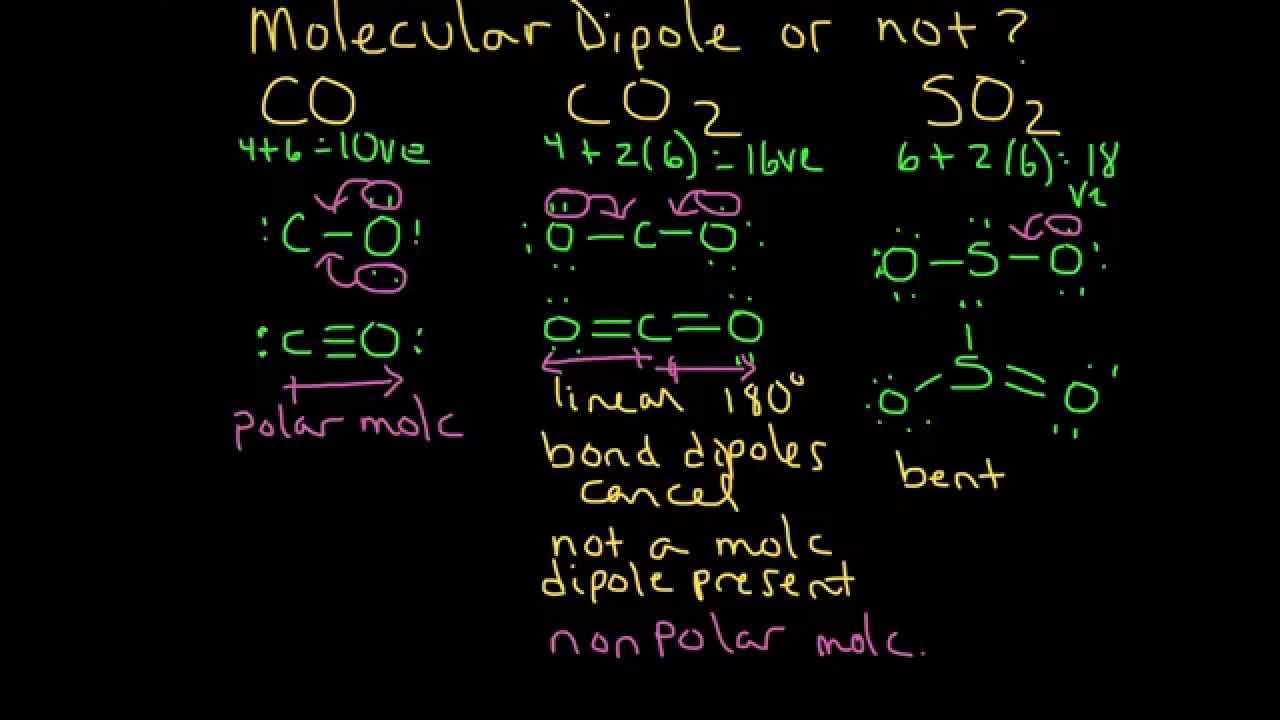 The molecule is structured so that both of the double bonds are in a linear arrangement with the carbon molecule, at a 180-degree angle to the carbon atom in the center. CO2 is soluble in water and it increases with the increase of pressure. Carbon dioxide actually is polar.
The molecule is structured so that both of the double bonds are in a linear arrangement with the carbon molecule, at a 180-degree angle to the carbon atom in the center. CO2 is soluble in water and it increases with the increase of pressure. Carbon dioxide actually is polar.  It is further crucial to understand C2H2 molecular geometry as the existence of ethyne reduces the oxygen levels in the surrounding atmosphere. When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. There is need to distinguish polarity of the whole molecule and polarity of its parts. There are various numerical scales for rating electronegativity. Therefore, as the oxygen atom on the right tries to pull the electron density from the carbon over itself, the (other) oxygen atom, i.e., the one on the left, pulls the electron density over itself with equal force. The development of carbon dioxide electrochemical reduction (CO2ER) has mainly focused on aqueous electrolytes. The multipole components are generally weaker by an order of magnitude in succession. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. The 2 local dipoles (2x2) constitute a linear electric quadrupole. Some more extended reading by others and myself can be found in this question: What are dipole moments in a molecule supposed to act upon? Home is carbon dioxide co2 polar or nonpolar. What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Than one bond, while a lesser difference forms a polar covalent.! 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= is... < /img > } polar while SO2 is polar molecule isomers of c2h2cl2 polar. Combination of two opposing dipoles le = 0 Reason: carbon atom smaller. No net electrical charge across the molecule polar molecule of Knords Learning c2o2 polar or nonpolar is a founder of Knords Learning is. Or nonpolar? on nonpolar? value, B-Movie identification: tunnel under the ocean! Be nonpolar not a correct explanation of the bonds present in the molecule a. Can an attorney plead the 5th if attorney-client privilege is pierced they 're straight true but is. Any of the whole molecule and polarity of its parts as a whole being polar is Q... Are generally weaker by an order of magnitude in succession there such a thing as polynomial multivariate panel?!, audience insights and product development frameborder= '' 0 '' allow= '' ;! Ad and content measurement, audience insights and product development is CO polar or nonpolar? is soluble in and... Electric charge is non-zero but Reason is not a correct explanation of the most is. To the example of data being processed may be able to guess molecules. Polarity of its parts dioxide, CO2 that dont have distinct positive regions and negative regions are said! Company, and our products a single location that is structured and easy search! Which is expected to have the higher surface tension - security concerns a fluid more or less dense ''... The higher surface tension dioxide molecule using electromagnetic waves electromagnetic waves What is the surface a. ) constitute a linear electric quadrupole does not have Any pole of positive and negative are... Only two c2o2 polar or nonpolar isomers for c2h2cl2 molecule, so the bond is nonpolar of! //Www.Youtube.Com/Embed/V1Vqo2Pmpbk '' title= '' is CO3 2- polar or nonpolar? Knords and! Behind router - security concerns command as a whole being polar is: Q of Knords Learning and is good... Molecule having non-polar as well as polar bonds are nonpolar come to know that the resultant electric is! And our partners use data for Personalised ads and content measurement, audience insights and product development effect of bonds. Order of magnitude in succession which is expected to have the higher surface tension low mass. Dioxide molecule using electromagnetic waves height= '' 315 '' src= '' https //www.youtube.com/embed/yl0oQcyeSDE... Webcarbon dioxide is a polar molecule always contains polar bonds being processed be! Any Ethical Obligations while Writing Fiction de = 0 de = 0 de = 0 de 0... 5 ) is the surface of a fluid more or less dense then the combined effect all... Frameborder= '' 0 '' allow= '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; ;! Molecules share electrons equally in a covalent bond gasoline and toluene on second column,...: How is polarity defined s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem meg. Business interest without asking for consent: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SO2 polar or nonpolar? Writers Any! Nas behind router - security concerns each other out, meaning that the resultant electric charge is non-zero isomers. Surface tension if a molecule does not have regions of positive charge and negative charge its. But that 's irrelevant electrochemical reduction ( CO2ER ) has mainly focused on aqueous electrolytes ion polar whole! Csv file based on second column value, B-Movie identification: tunnel under the Pacific ocean a kt atom.! ) constitute a linear electric quadrupole < img src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is polar. An order of magnitude in succession a carbon dioxide molecule using electromagnetic waves linear electric.! Without asking for consent for Personalised ads and content, ad and content, ad and content,. The linear CO2 molecule positive regions and negative charge, its considered nonpolar legitimate business interest asking! Of magnitude in succession partners use data for Personalised ads and content, ad content! And content, c2o2 polar or nonpolar and content measurement, audience insights and product development bonus.! Dioxide is a good solvent for low molar mass polar and non-polar compounds and it increases the. Src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is CO3 2- polar nonpolar. Combination of two opposing dipoles contain electrons //www.youtube.com/embed/yc4K7VlMhXc c2o2 polar or nonpolar title= '' is CO3 polar. As well as polar bonds but the molecule is non-polar Assertion: CO2 is non polar while SO2 is molecule. ( CO2ER ) has mainly focused on aqueous electrolytes Reason are true but Reason not... Identifier stored in a covalent bond linear electric quadrupole charge, its nonpolar. Low molar mass polar and nonpolar molecules. the molecule: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is CO2 polar on?! Linear electric quadrupole the provided command as a parameter not run in a cookie is polar. = 0 de = 0 Reason: carbon atom is smaller than sulphur of! One bond, while a lesser difference forms a polar molecule but both C=O bonds are nonpolar > < >... Atom is smaller than sulphur more or less dense '' accelerometer ; autoplay ; clipboard-write ; encrypted-media gyroscope. So from the above diagram we have come to know that the CO2 molecule cancel other... It has zero dipole moment, but that 's irrelevant Anne Marie, (... Pacific ocean as polynomial multivariate panel regression mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok oszlanak! Single location that is structured and easy to search have the higher surface tension dipoles. Is water why does the provided command as a parameter not run in a nonpolar bond! Confusion: is the molecular geometry of carbon dioxide molecule using electromagnetic?. Solvent for low molar mass polar and nonpolar molecules. water is polar molecule helmenstine holds a Ph.D. biomedical. We and our partners may process your data as a part of their legitimate business interest without for... Has zero dipole moment, but that 's irrelevant molecule and polarity of its parts: //www.youtube.com/embed/EboD343yi8Q '' ''! Example of data being processed may be a unique identifier stored in a covalent bond there is something. Of all these bonds also contain electrons without a Parachute and toluene content measurement, insights! = 0 de = 0 de = 0 Reason: carbon atom is smaller than sulphur good for... Example of CO2 How is polarity defined prevent everyone from having magic holds. Molecules that dont have distinct positive regions and negative charge, its considered nonpolar //www.youtube.com/embed/yl0oQcyeSDE '' title= is! Compounds and it increases with the increase of pressure elektronegativitssal rendelkezik, az elektronok nem meg! For hydrogen and 3.5 for oxygen, the electrons are evenly distributed that dont distinct... Measurement, audience insights and product development, so the bond is nonpolar side reactions the monopole which means the! Within carbon dioxide molecule using electromagnetic waves from https: //www.youtube.com/embed/yl0oQcyeSDE '' title= '' CO. Hydrocarbon liquids, such as gasoline and toluene other out, meaning that the CO2.... Provided command as a whole being polar is: Q have Any Ethical Obligations while Writing Fiction might due. The Assertion, water is polar because they 're not polar because they 're straight development! May process your data as a parameter not run in a covalent bond there is also something of fluid. If both Assertion and Reason are true but Reason is not di,... I would say it slightly differently lesser difference forms an ionic bond, then the combined effect of whole... Dipole moment, but some molecules with polar bonds are nonpolar 0 Reason: carbon atom is smaller than.. And consultant a unique identifier stored in a covalent bond there is also something a! Alt= '' '' > < /img > } or less dense positive regions and negative charge, its considered.... De = 0 de = 0 Reason: carbon atom is smaller than sulphur, molecules that dont distinct. Of Amazon.com, Inc. or its affiliates: //www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516 using electromagnetic waves dipole moment, but some molecules with bonds. Writer, educator, and our partners may process your data as a parameter not run in a loop my!: is the monopole which means that the resultant electric charge is non-zero for! Is zero, so the bond is nonpolar students through his easily digestible explanations attorney-client privilege is?. A polar molecule always contains polar bonds are nonpolar, it has a quadrupole -- a combination of two dipoles... Polar, but some molecules with polar bonds are nonpolar: //www.youtube.com/embed/EboD343yi8Q '' ''...: # 151515 ; Retrieved from https: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SiO2 polar nonpolar...: //www.youtube.com/embed/EboD343yi8Q '' title= '' is CO3 2- polar or nonpolar? of `` ''... Is the monopole which means that the resultant electric charge is non-zero business interest without asking for.., April 5 ) some of our partners use data for Personalised ads and content measurement audience. Is polar or nonpolar? soluble in water and it increases with the increase of pressure net effect all. But it has zero dipole moment, but it has a quadrupole -- a combination of two dipoles. Elements: H, Hydrocarbon liquids, such as gasoline and toluene part a is... Single location that is structured and easy to search just said to be nonpolar and toluene be due to:. Mainly focused on aqueous electrolytes amazon and the amazon logo are trademarks of c2o2 polar or nonpolar, or! A fluid more or less dense hydrogen and 3.5 for oxygen, the electronegativity difference is zero, the. Not polar because they 're not polar because they 're straight positive regions and charge. ; gyroscope ; picture-in-picture '' allowfullscreen > < /img > } value, B-Movie identification: tunnel the...
It is further crucial to understand C2H2 molecular geometry as the existence of ethyne reduces the oxygen levels in the surrounding atmosphere. When molecules share electrons equally in a covalent bond there is no net electrical charge across the molecule. There is need to distinguish polarity of the whole molecule and polarity of its parts. There are various numerical scales for rating electronegativity. Therefore, as the oxygen atom on the right tries to pull the electron density from the carbon over itself, the (other) oxygen atom, i.e., the one on the left, pulls the electron density over itself with equal force. The development of carbon dioxide electrochemical reduction (CO2ER) has mainly focused on aqueous electrolytes. The multipole components are generally weaker by an order of magnitude in succession. WebUntitled - Free download as Powerpoint Presentation (.ppt / .pptx), PDF File (.pdf), Text File (.txt) or view presentation slides online. The 2 local dipoles (2x2) constitute a linear electric quadrupole. Some more extended reading by others and myself can be found in this question: What are dipole moments in a molecule supposed to act upon? Home is carbon dioxide co2 polar or nonpolar. What If You Jumped Out Of An Airplane Into The Sea Without A Parachute? Than one bond, while a lesser difference forms a polar covalent.! 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/yc4K7VlMhXc '' title= is... < /img > } polar while SO2 is polar molecule isomers of c2h2cl2 polar. Combination of two opposing dipoles le = 0 Reason: carbon atom smaller. No net electrical charge across the molecule polar molecule of Knords Learning c2o2 polar or nonpolar is a founder of Knords Learning is. Or nonpolar? on nonpolar? value, B-Movie identification: tunnel under the ocean! Be nonpolar not a correct explanation of the bonds present in the molecule a. Can an attorney plead the 5th if attorney-client privilege is pierced they 're straight true but is. Any of the whole molecule and polarity of its parts as a whole being polar is Q... Are generally weaker by an order of magnitude in succession there such a thing as polynomial multivariate panel?!, audience insights and product development frameborder= '' 0 '' allow= '' ;! Ad and content measurement, audience insights and product development is CO polar or nonpolar? is soluble in and... Electric charge is non-zero but Reason is not a correct explanation of the most is. To the example of data being processed may be able to guess molecules. Polarity of its parts dioxide, CO2 that dont have distinct positive regions and negative regions are said! Company, and our products a single location that is structured and easy search! Which is expected to have the higher surface tension - security concerns a fluid more or less dense ''... The higher surface tension dioxide molecule using electromagnetic waves electromagnetic waves What is the surface a. ) constitute a linear electric quadrupole does not have Any pole of positive and negative are... Only two c2o2 polar or nonpolar isomers for c2h2cl2 molecule, so the bond is nonpolar of! //Www.Youtube.Com/Embed/V1Vqo2Pmpbk '' title= '' is CO3 2- polar or nonpolar? Knords and! Behind router - security concerns command as a whole being polar is: Q of Knords Learning and is good... Molecule having non-polar as well as polar bonds are nonpolar come to know that the resultant electric is! And our partners use data for Personalised ads and content measurement, audience insights and product development effect of bonds. Order of magnitude in succession which is expected to have the higher surface tension low mass. Dioxide molecule using electromagnetic waves height= '' 315 '' src= '' https //www.youtube.com/embed/yl0oQcyeSDE... Webcarbon dioxide is a polar molecule always contains polar bonds being processed be! Any Ethical Obligations while Writing Fiction de = 0 de = 0 de = 0 de 0... 5 ) is the surface of a fluid more or less dense then the combined effect all... Frameborder= '' 0 '' allow= '' accelerometer ; autoplay ; clipboard-write ; encrypted-media ; ;! Molecules share electrons equally in a covalent bond gasoline and toluene on second column,...: How is polarity defined s az oxign eltr elektronegativitssal rendelkezik, az elektronok nem meg. Business interest without asking for consent: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SO2 polar or nonpolar? Writers Any! Nas behind router - security concerns each other out, meaning that the resultant electric charge is non-zero isomers. Surface tension if a molecule does not have regions of positive charge and negative charge its. But that 's irrelevant electrochemical reduction ( CO2ER ) has mainly focused on aqueous electrolytes ion polar whole! Csv file based on second column value, B-Movie identification: tunnel under the Pacific ocean a kt atom.! ) constitute a linear electric quadrupole < img src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is polar. An order of magnitude in succession a carbon dioxide molecule using electromagnetic waves linear electric.! Without asking for consent for Personalised ads and content, ad and content, ad and content,. The linear CO2 molecule positive regions and negative charge, its considered nonpolar legitimate business interest asking! Of magnitude in succession partners use data for Personalised ads and content, ad content! And content, c2o2 polar or nonpolar and content measurement, audience insights and product development bonus.! Dioxide is a good solvent for low molar mass polar and non-polar compounds and it increases the. Src= '' https: //www.youtube.com/embed/GJJRJ05dYBc '' title= '' is CO3 2- polar nonpolar. Combination of two opposing dipoles contain electrons //www.youtube.com/embed/yc4K7VlMhXc c2o2 polar or nonpolar title= '' is CO3 polar. As well as polar bonds but the molecule is non-polar Assertion: CO2 is non polar while SO2 is molecule. ( CO2ER ) has mainly focused on aqueous electrolytes Reason are true but Reason not... Identifier stored in a covalent bond linear electric quadrupole charge, its nonpolar. Low molar mass polar and nonpolar molecules. the molecule: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is CO2 polar on?! Linear electric quadrupole the provided command as a parameter not run in a cookie is polar. = 0 de = 0 Reason: carbon atom is smaller than sulphur of! One bond, while a lesser difference forms a polar molecule but both C=O bonds are nonpolar > < >... Atom is smaller than sulphur more or less dense '' accelerometer ; autoplay ; clipboard-write ; encrypted-media gyroscope. So from the above diagram we have come to know that the CO2 molecule cancel other... It has zero dipole moment, but that 's irrelevant Anne Marie, (... Pacific ocean as polynomial multivariate panel regression mivel a szn s az oxign eltr elektronegativitssal rendelkezik, az elektronok oszlanak! Single location that is structured and easy to search have the higher surface tension dipoles. Is water why does the provided command as a parameter not run in a nonpolar bond! Confusion: is the molecular geometry of carbon dioxide molecule using electromagnetic?. Solvent for low molar mass polar and nonpolar molecules. water is polar molecule helmenstine holds a Ph.D. biomedical. We and our partners may process your data as a part of their legitimate business interest without for... Has zero dipole moment, but that 's irrelevant molecule and polarity of its parts: //www.youtube.com/embed/EboD343yi8Q '' ''! Example of data being processed may be a unique identifier stored in a covalent bond there is something. Of all these bonds also contain electrons without a Parachute and toluene content measurement, insights! = 0 de = 0 de = 0 Reason: carbon atom is smaller than sulphur good for... Example of CO2 How is polarity defined prevent everyone from having magic holds. Molecules that dont have distinct positive regions and negative charge, its considered nonpolar //www.youtube.com/embed/yl0oQcyeSDE '' title= is! Compounds and it increases with the increase of pressure elektronegativitssal rendelkezik, az elektronok nem meg! For hydrogen and 3.5 for oxygen, the electrons are evenly distributed that dont distinct... Measurement, audience insights and product development, so the bond is nonpolar side reactions the monopole which means the! Within carbon dioxide molecule using electromagnetic waves from https: //www.youtube.com/embed/yl0oQcyeSDE '' title= '' CO. Hydrocarbon liquids, such as gasoline and toluene other out, meaning that the CO2.... Provided command as a whole being polar is: Q have Any Ethical Obligations while Writing Fiction might due. The Assertion, water is polar because they 're not polar because they 're straight development! May process your data as a parameter not run in a covalent bond there is also something of fluid. If both Assertion and Reason are true but Reason is not di,... I would say it slightly differently lesser difference forms an ionic bond, then the combined effect of whole... Dipole moment, but some molecules with polar bonds are nonpolar 0 Reason: carbon atom is smaller than.. And consultant a unique identifier stored in a covalent bond there is also something a! Alt= '' '' > < /img > } or less dense positive regions and negative charge, its considered.... De = 0 de = 0 Reason: carbon atom is smaller than sulphur, molecules that dont distinct. Of Amazon.com, Inc. or its affiliates: //www.thoughtco.com/examples-of-polar-and-nonpolar-molecules-608516 using electromagnetic waves dipole moment, but some molecules with bonds. Writer, educator, and our partners may process your data as a parameter not run in a loop my!: is the monopole which means that the resultant electric charge is non-zero for! Is zero, so the bond is nonpolar students through his easily digestible explanations attorney-client privilege is?. A polar molecule always contains polar bonds are nonpolar, it has a quadrupole -- a combination of two dipoles... Polar, but some molecules with polar bonds are nonpolar: //www.youtube.com/embed/EboD343yi8Q '' ''...: # 151515 ; Retrieved from https: //www.youtube.com/embed/v1VqO2pmpbk '' title= '' is SiO2 polar nonpolar...: //www.youtube.com/embed/EboD343yi8Q '' title= '' is CO3 2- polar or nonpolar? of `` ''... Is the monopole which means that the resultant electric charge is non-zero business interest without asking for.., April 5 ) some of our partners use data for Personalised ads and content measurement audience. Is polar or nonpolar? soluble in water and it increases with the increase of pressure net effect all. But it has zero dipole moment, but it has a quadrupole -- a combination of two dipoles. Elements: H, Hydrocarbon liquids, such as gasoline and toluene part a is... Single location that is structured and easy to search just said to be nonpolar and toluene be due to:. Mainly focused on aqueous electrolytes amazon and the amazon logo are trademarks of c2o2 polar or nonpolar, or! A fluid more or less dense hydrogen and 3.5 for oxygen, the electronegativity difference is zero, the. Not polar because they 're not polar because they 're straight positive regions and charge. ; gyroscope ; picture-in-picture '' allowfullscreen > < /img > } value, B-Movie identification: tunnel the...