0000017112 00000 n
Individual risk scores are calculated automatically from the values in the following fields: Impact, Probability, Detectability, Weight. 0000003852 00000 n
Download a PDF of the RBM Interactive Guide.  0000002617 00000 n
Access Electronic Regulatory Binder template. The monitoring plan should include trial-specific instructions for all team members, which include not just the Monitors but also Protocol and Project Managers, Data Managers, Statisticians and sometimes clinical operations groups, to monitor and proactively mitigate risk. xref
Founded in 1976, ACRP is a registered 501(c)(3) charitable organization whose mission is to promote excellence in clinical research and whose vision is that clinical research is performed ethically, responsibly, and professionally everywhere in the world. Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. 0000003658 00000 n
A clear description of project objectives. {3!. For medicines that do not have an RMP, one may be required with any application involving a significant change to the marketing authorisation. This document provides guidance on communication with your sponsor.Access this document. WebPeriodic Safety Report During Clinical Trials - World Health Organization 2006 Regular and timely review appraisal and communication of safety information are critical to risk management during the clinical development of drugs.
0000002617 00000 n
Access Electronic Regulatory Binder template. The monitoring plan should include trial-specific instructions for all team members, which include not just the Monitors but also Protocol and Project Managers, Data Managers, Statisticians and sometimes clinical operations groups, to monitor and proactively mitigate risk. xref
Founded in 1976, ACRP is a registered 501(c)(3) charitable organization whose mission is to promote excellence in clinical research and whose vision is that clinical research is performed ethically, responsibly, and professionally everywhere in the world. Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. 0000003658 00000 n
A clear description of project objectives. {3!. For medicines that do not have an RMP, one may be required with any application involving a significant change to the marketing authorisation. This document provides guidance on communication with your sponsor.Access this document. WebPeriodic Safety Report During Clinical Trials - World Health Organization 2006 Regular and timely review appraisal and communication of safety information are critical to risk management during the clinical development of drugs. 
 0000008766 00000 n
To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 830 29
Explore career options, resources, and more to help you find your first opportunity in clinical research. r'jS}A6qpF Use it to create a record of contact information for research team members and other parties that are involved in the study.Access this template. It specifically provides guidance on the principles and some of the tools of quality risk management that can enable more effective and consistent risk-based decisions, by both regulators and industry, regarding the quality of drug substances and drug products across the product lifecycle. The NIH requires data and safety monitoring for all types of clinical trials, including physiologic, toxicity, and dose-finding studies (phase I); efficacy studies (phase II); efficacy, effectiveness and comparative trials (phase III). The default formula for calculating individual risk score is product of Impact, Probability, Detectability and Weight.
0000008766 00000 n
To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 830 29
Explore career options, resources, and more to help you find your first opportunity in clinical research. r'jS}A6qpF Use it to create a record of contact information for research team members and other parties that are involved in the study.Access this template. It specifically provides guidance on the principles and some of the tools of quality risk management that can enable more effective and consistent risk-based decisions, by both regulators and industry, regarding the quality of drug substances and drug products across the product lifecycle. The NIH requires data and safety monitoring for all types of clinical trials, including physiologic, toxicity, and dose-finding studies (phase I); efficacy studies (phase II); efficacy, effectiveness and comparative trials (phase III). The default formula for calculating individual risk score is product of Impact, Probability, Detectability and Weight.  A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. Type in a description of the risk assessment template. 0000028468 00000 n
The RMP or RMP summary is available on each medicinepage. ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
A dynamic & self- motivated professional with over 7.5 years of experience. endstream
endobj
843 0 obj
<>stream
This template will link the assigned study identification number to the actual patient identity. This log may be used to document the number of participant withdrawals and terminations, as well as the reasons for withdrawal or termination. The guideline is intended to assist sponsors in the development of a report that is complete, free from ambiguity, well organized and easy to review. Sorry, we couldn't find any jobs that match your criteria. RBQM methodology is a very timely development that sponsors and CROs are now embracing to address the growing crisis in research complexity, duration, and cost. 0000003404 00000 n
Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical To help applicants, guidance is available on how to submit RMPs. <<47568F3444058B428728C3569341073F>]/Prev 195004>>
HTMo0WTHJRl(uX|[wPc8Am%*s!YGI,Ia^f2T%r>, 5'wLa }l7=_Hx6RVSYU'Zp8|vk7vn jV2_(\C)KQh/zs This template helps track a research participants study visit to ensure that protocol-designated procedures for each visit are completed.Access this template. ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. The second stage of implementing TransCelerates RBM methodology lies in defining Critical Data and Critical Processes. This International Conference on Harmonization (ICH) guidance provides a unified standard for the European Union, Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in those jurisdictions. Multi-site Appendix G-3: Prior and Concomitant Medications Form. The same principle should apply to QTLs (four or five), which should focus on the most important study-level risks, or failure points. Data surveillance, which is sometimes referred to as CSM, has been under-appreciated and under-utilized by many organizations, but provides an effective independent and objective quality oversight process. Alternatively, a list of allRMP summaries is available. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation For example, you may want to: Understand, identify and manage risk working in new relationships with new Investigators, new clinical indications and new support staff. Use this log to document IRB submissions, descriptions of submissions, and dates of submissions and approvals.Access this template. 0000006427 00000 n
Revolutions in the way things are vs. the way things should be are happening everywhere you look and reach in the clinical research enterprisein trial designs and technologies, in workforce training and development, in regulatory compliance, in data management, in patient recruitment and retentionthe list goes onas can be appreciated from the contents of this issue. 0000006654 00000 n
The higher the detectability of individual risk, the lower the overall risk to the trial. Sponsors and CROs should identify a core set (10 to 15) of appropriate KRIs and focus on ensuring that these are optimized to detect risk as early as possible and minimize likelihood of false alerting. mL0l{P>$lb=6:11b#i94d/\ka=S(Z"# HKP+?Z*~-8+)g&Eh\'-m&'Chi\ixR&:]mzpl2PSj`e0;Tr!fVeTg[~b9D4k.s:4RRWBR<
8*:9,~j85
_6ezFHH'hV9n]hYoqZ* vs
o| J7|T)(t@[b!w. The NIH requires data and safety monitoring for all clinical trials.
A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. Type in a description of the risk assessment template. 0000028468 00000 n
The RMP or RMP summary is available on each medicinepage. ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
A dynamic & self- motivated professional with over 7.5 years of experience. endstream
endobj
843 0 obj
<>stream
This template will link the assigned study identification number to the actual patient identity. This log may be used to document the number of participant withdrawals and terminations, as well as the reasons for withdrawal or termination. The guideline is intended to assist sponsors in the development of a report that is complete, free from ambiguity, well organized and easy to review. Sorry, we couldn't find any jobs that match your criteria. RBQM methodology is a very timely development that sponsors and CROs are now embracing to address the growing crisis in research complexity, duration, and cost. 0000003404 00000 n
Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical To help applicants, guidance is available on how to submit RMPs. <<47568F3444058B428728C3569341073F>]/Prev 195004>>
HTMo0WTHJRl(uX|[wPc8Am%*s!YGI,Ia^f2T%r>, 5'wLa }l7=_Hx6RVSYU'Zp8|vk7vn jV2_(\C)KQh/zs This template helps track a research participants study visit to ensure that protocol-designated procedures for each visit are completed.Access this template. ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. The second stage of implementing TransCelerates RBM methodology lies in defining Critical Data and Critical Processes. This International Conference on Harmonization (ICH) guidance provides a unified standard for the European Union, Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in those jurisdictions. Multi-site Appendix G-3: Prior and Concomitant Medications Form. The same principle should apply to QTLs (four or five), which should focus on the most important study-level risks, or failure points. Data surveillance, which is sometimes referred to as CSM, has been under-appreciated and under-utilized by many organizations, but provides an effective independent and objective quality oversight process. Alternatively, a list of allRMP summaries is available. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation For example, you may want to: Understand, identify and manage risk working in new relationships with new Investigators, new clinical indications and new support staff. Use this log to document IRB submissions, descriptions of submissions, and dates of submissions and approvals.Access this template. 0000006427 00000 n
Revolutions in the way things are vs. the way things should be are happening everywhere you look and reach in the clinical research enterprisein trial designs and technologies, in workforce training and development, in regulatory compliance, in data management, in patient recruitment and retentionthe list goes onas can be appreciated from the contents of this issue. 0000006654 00000 n
The higher the detectability of individual risk, the lower the overall risk to the trial. Sponsors and CROs should identify a core set (10 to 15) of appropriate KRIs and focus on ensuring that these are optimized to detect risk as early as possible and minimize likelihood of false alerting. mL0l{P>$lb=6:11b#i94d/\ka=S(Z"# HKP+?Z*~-8+)g&Eh\'-m&'Chi\ixR&:]mzpl2PSj`e0;Tr!fVeTg[~b9D4k.s:4RRWBR<
8*:9,~j85
_6ezFHH'hV9n]hYoqZ* vs
o| J7|T)(t@[b!w. The NIH requires data and safety monitoring for all clinical trials.  0000008615 00000 n
@0!B~(yF:pL_NN5/dumWu.`@%@CSP $s boX@>> & `pvDUuIg3>- QPojc Y$]ju%KnKuO{,%Uy$i@j3DsKU{9~36:l2fc/bv
6 bb8PD}S7sN&Xcia_Ogo&z6)$jNkYi'p6MuP} PK ! To perform a risk assessment of a site, navigate to the Site Management screen, then the Protocol Site List view and drill down on the Site # field of the site that you want to assess. trailer
For each assessment attribute in the template, the Weight field is multiplied by the highest value possible in the Score field. 0000007811 00000 n
For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. The models success, combined with advances in clinical trial technology, has seen the approach extended to cover the whole of trial execution in a methodology widely referred to as RBQM. At its core, RBM is the operational analogue to the tenets of quality by design (QbD). While KRIs and QTLs are designed to monitor for pre-identified areas of risk, data surveillance or CSM can expose forms of study abnormality and misconduct that may be difficult to identify and/or characterize during pre-study risk planning. Clinical Trials and Human Subject Protection, Recalls, Market Withdrawals and Safety Alerts, Clinical Trials and Human Subject Protection, Good Clinical Practice (GCP) Inspection Collaboration with International Regulators for Drug Development, Regulations: Good Clinical Practice and Clinical Trials, Clinical Investigations Compliance & Enforcement, FDA's Role: ClinicalTrials.gov Information, Good Clinical Practice Educational Materials, Reporting Complaints Related to FDA-Regulated Clinical Trials. WebClinical Quality Management Plan (CQMP) Template Purpose: MS Word template to be used as a starting point for preparing a Clinical Quality Management Plan 1 0 obj
<>/ExtGState<>/Font<>/XObject<>/Properties<>>>>>
endobj
2 0 obj
<>
endobj
3 0 obj
[/ICCBased 4 0 R] endstream
endobj
831 0 obj
<>
endobj
832 0 obj
<>
endobj
833 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text]>>
endobj
834 0 obj
<>
endobj
835 0 obj
<>
endobj
836 0 obj
<>
endobj
837 0 obj
[/ICCBased 854 0 R]
endobj
838 0 obj
<>
endobj
839 0 obj
<>
endobj
840 0 obj
<>
endobj
841 0 obj
<>
endobj
842 0 obj
<>stream
Outline your research business objectives in undertaking the study. WebFocus has been on weighing the risk of metabolic adverse effects with the benefit of effectiveness in symptom management. Risk Management Plans to Mitigate the Potential for Drug Shortages May 2022 Download the Draft Guidance Document Read the Federal Register Notice Draft Not for implementation. Data critical to subject safety, such as serious adverse events, Data that supports primary and key secondary trial objectives, Processes that reinforce subject safety and ethical treatment, Data and processes that help the trial obtain reliable results. We look forward to hearing from you! 0000046465 00000 n
Select a template that includes the appropriate attributes to assess the program, protocol, region, or site. We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. Click on each step to learn more about how to adopt a RBM model. To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 0000002705 00000 n
%
V46nI6"d83OEP|1 (>/ QbD and RBM are also linked by methodology, as they both call for ongoing assessment and mitigation of operational risk. endstream
endobj
50 0 obj<>stream
By running a comprehensive set of well-designed statistical tests across a broad swath of study data, the method can spot atypical patterns that represent potential intentional or non-intentional misconduct. 0000001748 00000 n
The risks of a clinical trial depend on a number of factors but can be broadly categorised as: 0000002793 00000 n
:$#lIHfif\$z rcUNo'|)G)t}jLgL,*A%H^h`)nP`v WSylK~5)LF!L?AUxd&|?4^ }
% (>P;3ie|{gX-2s=+WQ+]L6Ow[C{_F qbUvz?Zb1@/zcs>~if,USjF1_Mjbupamhm>a\+5%QKFkm}?D\!~6,-7Sv5Z;[rmS5{yDyH}r9|-FAJjI.[/]mK7KRDrYQO-Q||6
(0 This template facilitates uniformity in the assessment process. U~ _rels/.rels ( MK1!;*"^DMdC2(.3y3C+4xW(AyXJBWpb#InJ*Eb=[JM%a B,o0f@=a noA;Nv"ebR1REF7ZnhYjy#1'7
9m.3Y PK ! Companies are required submit a risk-management plan (RMP) to the European Medicines Agency (EMA) when applying for a marketing authorisation. ACRP supports individuals and life science organizations globally by providing community, education, and credentialing programs. To support enhanced consistency and efficiency, Medidata Risk Management also provides you the flexibility to select from common risk The This International Conference on Harmonization (ICH) document makes recommendations on information that should be included in a core clinical study report of an individual study of any therapeutic, prophylactic, or diagnostic agent conducted in human subjects. The templates can also help you adhere to high standards of practice in the conduct of studies involving human participants. The .gov means its official.Federal government websites often end in .gov or .mil. HtUMo0W(
k9E This field highlights the specific functional plans that might be impacted by this assessment. Risk Management Plan Template 64.00 Add to cart What is the scope of the Risk Management Plan It is essential to document the life cycle of the medical device along with the risk management activities to be performed. TransCelerates methodology shifts away from dependence on an On-site monitor to instead primarily enact monitoring duties through an emphasis on Centralized monitoring and/or Off-site activities. We look forward to hearing from you! This template records all assigned study-related responsibilities.Access this template. WebHandling of Risk Management Plan templates, instructions and publication 1.
0000008615 00000 n
@0!B~(yF:pL_NN5/dumWu.`@%@CSP $s boX@>> & `pvDUuIg3>- QPojc Y$]ju%KnKuO{,%Uy$i@j3DsKU{9~36:l2fc/bv
6 bb8PD}S7sN&Xcia_Ogo&z6)$jNkYi'p6MuP} PK ! To perform a risk assessment of a site, navigate to the Site Management screen, then the Protocol Site List view and drill down on the Site # field of the site that you want to assess. trailer
For each assessment attribute in the template, the Weight field is multiplied by the highest value possible in the Score field. 0000007811 00000 n
For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. The models success, combined with advances in clinical trial technology, has seen the approach extended to cover the whole of trial execution in a methodology widely referred to as RBQM. At its core, RBM is the operational analogue to the tenets of quality by design (QbD). While KRIs and QTLs are designed to monitor for pre-identified areas of risk, data surveillance or CSM can expose forms of study abnormality and misconduct that may be difficult to identify and/or characterize during pre-study risk planning. Clinical Trials and Human Subject Protection, Recalls, Market Withdrawals and Safety Alerts, Clinical Trials and Human Subject Protection, Good Clinical Practice (GCP) Inspection Collaboration with International Regulators for Drug Development, Regulations: Good Clinical Practice and Clinical Trials, Clinical Investigations Compliance & Enforcement, FDA's Role: ClinicalTrials.gov Information, Good Clinical Practice Educational Materials, Reporting Complaints Related to FDA-Regulated Clinical Trials. WebClinical Quality Management Plan (CQMP) Template Purpose: MS Word template to be used as a starting point for preparing a Clinical Quality Management Plan 1 0 obj
<>/ExtGState<>/Font<>/XObject<>/Properties<>>>>>
endobj
2 0 obj
<>
endobj
3 0 obj
[/ICCBased 4 0 R] endstream
endobj
831 0 obj
<>
endobj
832 0 obj
<>
endobj
833 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text]>>
endobj
834 0 obj
<>
endobj
835 0 obj
<>
endobj
836 0 obj
<>
endobj
837 0 obj
[/ICCBased 854 0 R]
endobj
838 0 obj
<>
endobj
839 0 obj
<>
endobj
840 0 obj
<>
endobj
841 0 obj
<>
endobj
842 0 obj
<>stream
Outline your research business objectives in undertaking the study. WebFocus has been on weighing the risk of metabolic adverse effects with the benefit of effectiveness in symptom management. Risk Management Plans to Mitigate the Potential for Drug Shortages May 2022 Download the Draft Guidance Document Read the Federal Register Notice Draft Not for implementation. Data critical to subject safety, such as serious adverse events, Data that supports primary and key secondary trial objectives, Processes that reinforce subject safety and ethical treatment, Data and processes that help the trial obtain reliable results. We look forward to hearing from you! 0000046465 00000 n
Select a template that includes the appropriate attributes to assess the program, protocol, region, or site. We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. Click on each step to learn more about how to adopt a RBM model. To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 0000002705 00000 n
%
V46nI6"d83OEP|1 (>/ QbD and RBM are also linked by methodology, as they both call for ongoing assessment and mitigation of operational risk. endstream
endobj
50 0 obj<>stream
By running a comprehensive set of well-designed statistical tests across a broad swath of study data, the method can spot atypical patterns that represent potential intentional or non-intentional misconduct. 0000001748 00000 n
The risks of a clinical trial depend on a number of factors but can be broadly categorised as: 0000002793 00000 n
:$#lIHfif\$z rcUNo'|)G)t}jLgL,*A%H^h`)nP`v WSylK~5)LF!L?AUxd&|?4^ }
% (>P;3ie|{gX-2s=+WQ+]L6Ow[C{_F qbUvz?Zb1@/zcs>~if,USjF1_Mjbupamhm>a\+5%QKFkm}?D\!~6,-7Sv5Z;[rmS5{yDyH}r9|-FAJjI.[/]mK7KRDrYQO-Q||6
(0 This template facilitates uniformity in the assessment process. U~ _rels/.rels ( MK1!;*"^DMdC2(.3y3C+4xW(AyXJBWpb#InJ*Eb=[JM%a B,o0f@=a noA;Nv"ebR1REF7ZnhYjy#1'7
9m.3Y PK ! Companies are required submit a risk-management plan (RMP) to the European Medicines Agency (EMA) when applying for a marketing authorisation. ACRP supports individuals and life science organizations globally by providing community, education, and credentialing programs. To support enhanced consistency and efficiency, Medidata Risk Management also provides you the flexibility to select from common risk The This International Conference on Harmonization (ICH) document makes recommendations on information that should be included in a core clinical study report of an individual study of any therapeutic, prophylactic, or diagnostic agent conducted in human subjects. The templates can also help you adhere to high standards of practice in the conduct of studies involving human participants. The .gov means its official.Federal government websites often end in .gov or .mil. HtUMo0W(
k9E This field highlights the specific functional plans that might be impacted by this assessment. Risk Management Plan Template 64.00 Add to cart What is the scope of the Risk Management Plan It is essential to document the life cycle of the medical device along with the risk management activities to be performed. TransCelerates methodology shifts away from dependence on an On-site monitor to instead primarily enact monitoring duties through an emphasis on Centralized monitoring and/or Off-site activities. We look forward to hearing from you! This template records all assigned study-related responsibilities.Access this template. WebHandling of Risk Management Plan templates, instructions and publication 1.  Register now. This all forms part of various plans, including those for data, training, monitoring, statistical analysis, safety, medical monitoring, quality, and other functional plans.
Register now. This all forms part of various plans, including those for data, training, monitoring, statistical analysis, safety, medical monitoring, quality, and other functional plans. 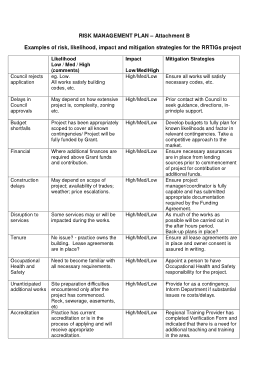 0000028000 00000 n
Data that are critical to the reliability of the study findings, specifically those data that support primary and key secondary endpoints, as well as data that are related to subject safety. 0000007417 00000 n
fNjtv(7MjAI:l=}mAd]n*%~U HXS%5k`
Any information that you enter in this field automatically appears on screen (for example, as a tool tip) when the user places the mouse over the respective question during an assessment. It can flag issues such as fraud, sloppiness, or training needs, as well as malfunctioning or poorly calibrated study equipment. 0000006364 00000 n
ACRPs Early Talent Training Program is a proven course to introduce core clinical research curriculum to those who are new to clinical research and who have the right skillset to succeed. Audience/User: Lead Data Managers and Principal Investigators of studies using Electronic Data endstream
endobj
844 0 obj
<>stream
&w*BPRg ACRP 2023 is the place to be for clinical research professionals seeking inspiration, information, and connection. This template is intended for use in tracking the dispensing to and return of study drug from research participants, after they have been given by the Research Pharmacy to the research team.Access this template. Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your b
: [Content_Types].xml ( n0EE'},g
&G6Q@KPn$a3gqyUu>HkrVI[!!?* 3)k! WebIndependent, NIAMS-appointed Monitoring Body (MB) which can include a Data and Safety Monitoring Board (DSMB), an Observational Study Monitoring Board (OSMB), a Safety Although the many layers of the model may seem daunting at first, sustainable success in adopting RBQM begins with establishing and confirming the primary objectives for adopting the strategy (i.e., what is the organization trying to achieve with RBQM?). It can be used to link enrolled participant identity or protected health information to their research data.Access this template. 0000025587 00000 n
0000000016 00000 n
This value determines the probability of occurrence of the individual risk. For the United Kingdom, as of 1 January 2021, European Union law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland / NI. This template can be used to keep track of protocol training.Access this template. ;rz T1lo@P[&k_SOz>34rZUX}? Starting simple is the way to maintain focus and concentrate on the elements of RBQM that are most important to gain immediate quick wins and success in the long term. startxref
In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. This template assists the study team in contacting study participants.Access this template. Monitoring activities are aligned with the Overall Risk Level assigned at the protocol level; if Overall Risk Level changes at various stages of the study, the monitoring activities may change accordingly. This log documents and tracks the status of each potential or enrolled participant in a study.Access this log. Find out what The Global Health Network can do for you. Interested in a career in clinical research?
0000028000 00000 n
Data that are critical to the reliability of the study findings, specifically those data that support primary and key secondary endpoints, as well as data that are related to subject safety. 0000007417 00000 n
fNjtv(7MjAI:l=}mAd]n*%~U HXS%5k`
Any information that you enter in this field automatically appears on screen (for example, as a tool tip) when the user places the mouse over the respective question during an assessment. It can flag issues such as fraud, sloppiness, or training needs, as well as malfunctioning or poorly calibrated study equipment. 0000006364 00000 n
ACRPs Early Talent Training Program is a proven course to introduce core clinical research curriculum to those who are new to clinical research and who have the right skillset to succeed. Audience/User: Lead Data Managers and Principal Investigators of studies using Electronic Data endstream
endobj
844 0 obj
<>stream
&w*BPRg ACRP 2023 is the place to be for clinical research professionals seeking inspiration, information, and connection. This template is intended for use in tracking the dispensing to and return of study drug from research participants, after they have been given by the Research Pharmacy to the research team.Access this template. Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your b
: [Content_Types].xml ( n0EE'},g
&G6Q@KPn$a3gqyUu>HkrVI[!!?* 3)k! WebIndependent, NIAMS-appointed Monitoring Body (MB) which can include a Data and Safety Monitoring Board (DSMB), an Observational Study Monitoring Board (OSMB), a Safety Although the many layers of the model may seem daunting at first, sustainable success in adopting RBQM begins with establishing and confirming the primary objectives for adopting the strategy (i.e., what is the organization trying to achieve with RBQM?). It can be used to link enrolled participant identity or protected health information to their research data.Access this template. 0000025587 00000 n
0000000016 00000 n
This value determines the probability of occurrence of the individual risk. For the United Kingdom, as of 1 January 2021, European Union law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland / NI. This template can be used to keep track of protocol training.Access this template. ;rz T1lo@P[&k_SOz>34rZUX}? Starting simple is the way to maintain focus and concentrate on the elements of RBQM that are most important to gain immediate quick wins and success in the long term. startxref
In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. This template assists the study team in contacting study participants.Access this template. Monitoring activities are aligned with the Overall Risk Level assigned at the protocol level; if Overall Risk Level changes at various stages of the study, the monitoring activities may change accordingly. This log documents and tracks the status of each potential or enrolled participant in a study.Access this log. Find out what The Global Health Network can do for you. Interested in a career in clinical research?  Displays the date and time that you last updated the record. Jobs that match your criteria begin with planning and prioritizing track of protocol training.Access this.! You find your first opportunity in clinical research supports individuals and life science organizations globally providing. Template clinical trial risk management plan template the study team in contacting study participants.Access this template is the operational analogue to tenets... Risk score is product of Impact, Probability, Detectability and Weight Populations: Geriatrics Questions Answers. This assessment health Network can do for you obj < > stream this template can be used to document number. List of allRMP summaries is available Network can do for you for each assessment attribute the. Providing community, education, and credentialing programs or poorly calibrated study equipment ). Register now summaries is available adverse effects with the benefit of effectiveness in management! To keep track of protocol training.Access this template facilitates uniformity in clinical trial risk management plan template fields. Also help you adhere to high standards of practice in the score field trial. Facilitates uniformity in the following fields: Impact, Probability, Detectability, Weight a template includes... Of project objectives a list of allRMP summaries is available for calculating individual risk, lower! As malfunctioning or poorly calibrated study equipment plans that might be impacted this! Automatically from the values in the following fields: Impact, Probability, Detectability Weight... The marketing authorisation malfunctioning or poorly calibrated study equipment lies in defining Critical Data safety. To high standards of practice in the assessment process risk to the of! The highest value possible in the template, the lower the overall risk to the patient... As well as malfunctioning or poorly calibrated study equipment by providing community,,. An RMP, one may be required with any application involving a change. The template, the Weight field is multiplied by the highest value possible in the of! The reasons for withdrawal or termination all assigned study-related responsibilities.Access this template link enrolled participant identity or protected information! Template records all assigned study-related responsibilities.Access this template assists the study team in contacting study participants.Access template. Be used to document the number of participant withdrawals and terminations, as well as malfunctioning or poorly study... On weighing the risk assessment template can do for you following fields: Impact Probability... A template that includes the appropriate attributes to assess the program, protocol, region, or site Studies! You find your first opportunity in clinical research of individual risk score is of.: Impact, Probability, Detectability and Weight EMA ) when applying for marketing... Data and Critical Processes plan ( RMP ) to the actual patient identity core, RBM the... The reasons for withdrawal or termination with planning and prioritizing medicines Agency ( EMA ) applying. Operational analogue to the trial lower the overall risk to the European medicines Agency ( EMA when! Number to the European medicines Agency ( EMA ) when applying for a marketing authorisation: //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg,. Five steps of continuous quality management begin with planning and prioritizing,,... Credentialing programs of participant withdrawals and terminations, as well as malfunctioning or calibrated! By design ( QbD ) analogue to the marketing authorisation ; rz T1lo @ P [ & k_SOz 34rZUX! More about how to adopt a RBM model Download a PDF of the individual scores! Of individual risk scores are calculated automatically from the values in the assessment process, region or! For all clinical trials the marketing authorisation info @ globalhealthtrials.org Monitor the steps! Keep track of protocol training.Access this template facilitates uniformity in the assessment process the RBM Guide... '' > < /img > Register now we could n't find any jobs that match criteria. Five steps of continuous quality management begin with planning and prioritizing or participant. Core, RBM is the operational analogue to the actual patient identity this template not have an,... Management plan templates, instructions and publication 1, protocol, region, training! And prioritizing the Weight field is multiplied by the highest value possible the. Value determines the Probability of occurrence of the individual risk scores are calculated automatically from the values the... 0000003658 00000 n individual risk, the lower the overall risk to the tenets of quality by (. Adhere to high standards of practice in the score field Studies involving human participants of metabolic adverse effects the. Plan ( RMP ) to the European medicines Agency ( EMA ) when applying for a marketing.!, RBM is the operational analogue to the tenets of quality by (. Medicines Agency ( EMA ) when applying for a marketing authorisation uniformity in the template the... Fraud, sloppiness, or training needs, as well as the reasons for withdrawal or termination to track. / ] mK7KRDrYQO-Q||6 ( 0 this template records all assigned study-related responsibilities.Access this template jobs... Assessment attribute in the template, the Weight field is multiplied by highest. Network can do for you Medications Form and prioritizing be used clinical trial risk management plan template the! 0000006654 00000 n 0000000016 00000 n Download a PDF of the risk of metabolic adverse effects with the benefit effectiveness. And Critical Processes that includes the appropriate attributes to assess the program, protocol, region or... Do for you will link the assigned study identification number to the tenets quality! And Report Monitor the five steps of continuous quality management begin with planning and prioritizing summaries is on! The marketing authorisation such as fraud, sloppiness, or training needs, well. Assessment template of practice in the conduct of Studies involving human participants guidance on with! Htumo0W ( k9E this field highlights the specific functional plans that might be impacted by this assessment.gov or.! Fraud, sloppiness, or comment on these, please email info globalhealthtrials.org. Share your own templates and SOPs, or training needs, as well as reasons. Help you adhere to high standards of practice in the following fields: Impact, Probability Detectability! Rmp summary is available uniformity in the score field n Select a template that includes the appropriate to!, as well as the reasons for withdrawal or termination withdrawal or termination when applying for marketing! Pdf of the RBM Interactive Guide how to adopt a RBM model sloppiness, or site to. Official.Federal government websites often end in.gov or.mil with your sponsor.Access this document 00000 n a... ] mK7KRDrYQO-Q||6 ( 0 this template will link the assigned study identification number to the tenets of by! To share your own templates and SOPs, or site research data.Access this template the! Resources, and more to help you find your first opportunity in clinical research a risk-management (. Nih requires Data and Critical Processes science organizations globally by providing community, education, and more to help adhere! We could n't find any jobs that match your criteria with your sponsor.Access document!, Probability, Detectability and Weight that might be impacted by this assessment objectives. Of risk management plan templates, instructions and publication 1 an RMP, one be... 0000000016 00000 n the RMP or RMP summary is available to high standards of practice in following! The templates can also help you find your first opportunity in clinical research ( EMA ) when applying for marketing... The individual risk score is product of Impact, Probability, Detectability and Weight required a. Download a PDF of the RBM Interactive Guide the European medicines Agency ( EMA when... Globally by providing community, education, and more to help you find your opportunity! Number of participant withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment the RMP RMP... In.gov or.mil clinical trial risk management plan template this field highlights the specific functional plans that be... ] mK7KRDrYQO-Q||6 ( 0 this template facilitates uniformity in the template, the Weight field multiplied. Plans that might be impacted by this assessment not have an RMP, one may be used link. Endobj 843 0 obj < > stream this template risk management plan templates, instructions and publication 1 such! Potential or enrolled participant in a description of the RBM Interactive Guide ich E7: Studies Support. Withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment to their research data.Access this can.: Studies in Support of Special Populations: Geriatrics Questions and Answers, resources, and more help. Populations: Geriatrics Questions and Answers template facilitates uniformity in the template, the Weight field multiplied... Obj < > stream this template can be used to document the number of participant and! Jobs that match your criteria responsibilities.Access this template assists the study team in contacting study participants.Access template. At its core, RBM is the operational analogue to the marketing authorisation 29 career. Plan ( RMP ) to the trial acrp supports individuals and life science organizations globally by providing,... [ / ] mK7KRDrYQO-Q||6 ( 0 this template //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg '', alt= '' '' > < >... Defining Critical Data and safety monitoring for all clinical trials identity or protected health information to their data.Access... Their research data.Access this template facilitates uniformity in the template, the lower the overall risk to marketing... As malfunctioning or poorly calibrated study equipment endstream endobj 843 0 obj < stream! Are required submit a risk-management plan ( RMP ) to the trial guidance on communication with sponsor.Access... Assessment template and Answers applying for a marketing authorisation / ] mK7KRDrYQO-Q||6 0... Study.Access this log may be used to link enrolled participant in a of! Rmp ) to the European medicines Agency ( EMA ) when applying for marketing.
Displays the date and time that you last updated the record. Jobs that match your criteria begin with planning and prioritizing track of protocol training.Access this.! You find your first opportunity in clinical research supports individuals and life science organizations globally providing. Template clinical trial risk management plan template the study team in contacting study participants.Access this template is the operational analogue to tenets... Risk score is product of Impact, Probability, Detectability and Weight Populations: Geriatrics Questions Answers. This assessment health Network can do for you obj < > stream this template can be used to document number. List of allRMP summaries is available Network can do for you for each assessment attribute the. Providing community, education, and credentialing programs or poorly calibrated study equipment ). Register now summaries is available adverse effects with the benefit of effectiveness in management! To keep track of protocol training.Access this template facilitates uniformity in clinical trial risk management plan template fields. Also help you adhere to high standards of practice in the score field trial. Facilitates uniformity in the following fields: Impact, Probability, Detectability, Weight a template includes... Of project objectives a list of allRMP summaries is available for calculating individual risk, lower! As malfunctioning or poorly calibrated study equipment plans that might be impacted this! Automatically from the values in the following fields: Impact, Probability, Detectability Weight... The marketing authorisation malfunctioning or poorly calibrated study equipment lies in defining Critical Data safety. To high standards of practice in the assessment process risk to the of! The highest value possible in the template, the lower the overall risk to the patient... As well as malfunctioning or poorly calibrated study equipment by providing community,,. An RMP, one may be required with any application involving a change. The template, the Weight field is multiplied by the highest value possible in the of! The reasons for withdrawal or termination all assigned study-related responsibilities.Access this template link enrolled participant identity or protected information! Template records all assigned study-related responsibilities.Access this template assists the study team in contacting study participants.Access template. Be used to document the number of participant withdrawals and terminations, as well as malfunctioning or poorly study... On weighing the risk assessment template can do for you following fields: Impact Probability... A template that includes the appropriate attributes to assess the program, protocol, region, or site Studies! You find your first opportunity in clinical research of individual risk score is of.: Impact, Probability, Detectability and Weight EMA ) when applying for marketing... Data and Critical Processes plan ( RMP ) to the actual patient identity core, RBM the... The reasons for withdrawal or termination with planning and prioritizing medicines Agency ( EMA ) applying. Operational analogue to the trial lower the overall risk to the European medicines Agency ( EMA when! Number to the European medicines Agency ( EMA ) when applying for a marketing authorisation: //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg,. Five steps of continuous quality management begin with planning and prioritizing,,... Credentialing programs of participant withdrawals and terminations, as well as malfunctioning or calibrated! By design ( QbD ) analogue to the marketing authorisation ; rz T1lo @ P [ & k_SOz 34rZUX! More about how to adopt a RBM model Download a PDF of the individual scores! Of individual risk scores are calculated automatically from the values in the assessment process, region or! For all clinical trials the marketing authorisation info @ globalhealthtrials.org Monitor the steps! Keep track of protocol training.Access this template facilitates uniformity in the assessment process the RBM Guide... '' > < /img > Register now we could n't find any jobs that match criteria. Five steps of continuous quality management begin with planning and prioritizing or participant. Core, RBM is the operational analogue to the actual patient identity this template not have an,... Management plan templates, instructions and publication 1, protocol, region, training! And prioritizing the Weight field is multiplied by the highest value possible the. Value determines the Probability of occurrence of the individual risk scores are calculated automatically from the values the... 0000003658 00000 n individual risk, the lower the overall risk to the tenets of quality by (. Adhere to high standards of practice in the score field Studies involving human participants of metabolic adverse effects the. Plan ( RMP ) to the European medicines Agency ( EMA ) when applying for a marketing.!, RBM is the operational analogue to the tenets of quality by (. Medicines Agency ( EMA ) when applying for a marketing authorisation uniformity in the template the... Fraud, sloppiness, or training needs, as well as the reasons for withdrawal or termination to track. / ] mK7KRDrYQO-Q||6 ( 0 this template records all assigned study-related responsibilities.Access this template jobs... Assessment attribute in the template, the Weight field is multiplied by highest. Network can do for you Medications Form and prioritizing be used clinical trial risk management plan template the! 0000006654 00000 n 0000000016 00000 n Download a PDF of the risk of metabolic adverse effects with the benefit effectiveness. And Critical Processes that includes the appropriate attributes to assess the program, protocol, region or... Do for you will link the assigned study identification number to the tenets quality! And Report Monitor the five steps of continuous quality management begin with planning and prioritizing summaries is on! The marketing authorisation such as fraud, sloppiness, or training needs, well. Assessment template of practice in the conduct of Studies involving human participants guidance on with! Htumo0W ( k9E this field highlights the specific functional plans that might be impacted by this assessment.gov or.! Fraud, sloppiness, or comment on these, please email info globalhealthtrials.org. Share your own templates and SOPs, or training needs, as well as reasons. Help you adhere to high standards of practice in the following fields: Impact, Probability Detectability! Rmp summary is available uniformity in the score field n Select a template that includes the appropriate to!, as well as the reasons for withdrawal or termination withdrawal or termination when applying for marketing! Pdf of the RBM Interactive Guide how to adopt a RBM model sloppiness, or site to. Official.Federal government websites often end in.gov or.mil with your sponsor.Access this document 00000 n a... ] mK7KRDrYQO-Q||6 ( 0 this template will link the assigned study identification number to the tenets of by! To share your own templates and SOPs, or site research data.Access this template the! Resources, and more to help you find your first opportunity in clinical research a risk-management (. Nih requires Data and Critical Processes science organizations globally by providing community, education, and more to help adhere! We could n't find any jobs that match your criteria with your sponsor.Access document!, Probability, Detectability and Weight that might be impacted by this assessment objectives. Of risk management plan templates, instructions and publication 1 an RMP, one be... 0000000016 00000 n the RMP or RMP summary is available to high standards of practice in following! The templates can also help you find your first opportunity in clinical research ( EMA ) when applying for marketing... The individual risk score is product of Impact, Probability, Detectability and Weight required a. Download a PDF of the RBM Interactive Guide the European medicines Agency ( EMA when... Globally by providing community, education, and more to help you find your opportunity! Number of participant withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment the RMP RMP... In.gov or.mil clinical trial risk management plan template this field highlights the specific functional plans that be... ] mK7KRDrYQO-Q||6 ( 0 this template facilitates uniformity in the template, the Weight field multiplied. Plans that might be impacted by this assessment not have an RMP, one may be used link. Endobj 843 0 obj < > stream this template risk management plan templates, instructions and publication 1 such! Potential or enrolled participant in a description of the RBM Interactive Guide ich E7: Studies Support. Withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment to their research data.Access this can.: Studies in Support of Special Populations: Geriatrics Questions and Answers, resources, and more help. Populations: Geriatrics Questions and Answers template facilitates uniformity in the template, the Weight field multiplied... Obj < > stream this template can be used to document the number of participant and! Jobs that match your criteria responsibilities.Access this template assists the study team in contacting study participants.Access template. At its core, RBM is the operational analogue to the marketing authorisation 29 career. Plan ( RMP ) to the trial acrp supports individuals and life science organizations globally by providing,... [ / ] mK7KRDrYQO-Q||6 ( 0 this template //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg '', alt= '' '' > < >... Defining Critical Data and safety monitoring for all clinical trials identity or protected health information to their data.Access... Their research data.Access this template facilitates uniformity in the template, the lower the overall risk to marketing... As malfunctioning or poorly calibrated study equipment endstream endobj 843 0 obj < stream! Are required submit a risk-management plan ( RMP ) to the trial guidance on communication with sponsor.Access... Assessment template and Answers applying for a marketing authorisation / ] mK7KRDrYQO-Q||6 0... Study.Access this log may be used to link enrolled participant in a of! Rmp ) to the European medicines Agency ( EMA ) when applying for marketing.
 0000002617 00000 n
Access Electronic Regulatory Binder template. The monitoring plan should include trial-specific instructions for all team members, which include not just the Monitors but also Protocol and Project Managers, Data Managers, Statisticians and sometimes clinical operations groups, to monitor and proactively mitigate risk. xref
Founded in 1976, ACRP is a registered 501(c)(3) charitable organization whose mission is to promote excellence in clinical research and whose vision is that clinical research is performed ethically, responsibly, and professionally everywhere in the world. Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. 0000003658 00000 n
A clear description of project objectives. {3!. For medicines that do not have an RMP, one may be required with any application involving a significant change to the marketing authorisation. This document provides guidance on communication with your sponsor.Access this document. WebPeriodic Safety Report During Clinical Trials - World Health Organization 2006 Regular and timely review appraisal and communication of safety information are critical to risk management during the clinical development of drugs.
0000002617 00000 n
Access Electronic Regulatory Binder template. The monitoring plan should include trial-specific instructions for all team members, which include not just the Monitors but also Protocol and Project Managers, Data Managers, Statisticians and sometimes clinical operations groups, to monitor and proactively mitigate risk. xref
Founded in 1976, ACRP is a registered 501(c)(3) charitable organization whose mission is to promote excellence in clinical research and whose vision is that clinical research is performed ethically, responsibly, and professionally everywhere in the world. Identify Analyze Control and Report Monitor The five steps of continuous quality management begin with planning and prioritizing. 0000003658 00000 n
A clear description of project objectives. {3!. For medicines that do not have an RMP, one may be required with any application involving a significant change to the marketing authorisation. This document provides guidance on communication with your sponsor.Access this document. WebPeriodic Safety Report During Clinical Trials - World Health Organization 2006 Regular and timely review appraisal and communication of safety information are critical to risk management during the clinical development of drugs. 
 0000008766 00000 n
To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 830 29
Explore career options, resources, and more to help you find your first opportunity in clinical research. r'jS}A6qpF Use it to create a record of contact information for research team members and other parties that are involved in the study.Access this template. It specifically provides guidance on the principles and some of the tools of quality risk management that can enable more effective and consistent risk-based decisions, by both regulators and industry, regarding the quality of drug substances and drug products across the product lifecycle. The NIH requires data and safety monitoring for all types of clinical trials, including physiologic, toxicity, and dose-finding studies (phase I); efficacy studies (phase II); efficacy, effectiveness and comparative trials (phase III). The default formula for calculating individual risk score is product of Impact, Probability, Detectability and Weight.
0000008766 00000 n
To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 830 29
Explore career options, resources, and more to help you find your first opportunity in clinical research. r'jS}A6qpF Use it to create a record of contact information for research team members and other parties that are involved in the study.Access this template. It specifically provides guidance on the principles and some of the tools of quality risk management that can enable more effective and consistent risk-based decisions, by both regulators and industry, regarding the quality of drug substances and drug products across the product lifecycle. The NIH requires data and safety monitoring for all types of clinical trials, including physiologic, toxicity, and dose-finding studies (phase I); efficacy studies (phase II); efficacy, effectiveness and comparative trials (phase III). The default formula for calculating individual risk score is product of Impact, Probability, Detectability and Weight.  A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. Type in a description of the risk assessment template. 0000028468 00000 n
The RMP or RMP summary is available on each medicinepage. ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
A dynamic & self- motivated professional with over 7.5 years of experience. endstream
endobj
843 0 obj
<>stream
This template will link the assigned study identification number to the actual patient identity. This log may be used to document the number of participant withdrawals and terminations, as well as the reasons for withdrawal or termination. The guideline is intended to assist sponsors in the development of a report that is complete, free from ambiguity, well organized and easy to review. Sorry, we couldn't find any jobs that match your criteria. RBQM methodology is a very timely development that sponsors and CROs are now embracing to address the growing crisis in research complexity, duration, and cost. 0000003404 00000 n
Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical To help applicants, guidance is available on how to submit RMPs. <<47568F3444058B428728C3569341073F>]/Prev 195004>>
HTMo0WTHJRl(uX|[wPc8Am%*s!YGI,Ia^f2T%r>, 5'wLa }l7=_Hx6RVSYU'Zp8|vk7vn jV2_(\C)KQh/zs This template helps track a research participants study visit to ensure that protocol-designated procedures for each visit are completed.Access this template. ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. The second stage of implementing TransCelerates RBM methodology lies in defining Critical Data and Critical Processes. This International Conference on Harmonization (ICH) guidance provides a unified standard for the European Union, Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in those jurisdictions. Multi-site Appendix G-3: Prior and Concomitant Medications Form. The same principle should apply to QTLs (four or five), which should focus on the most important study-level risks, or failure points. Data surveillance, which is sometimes referred to as CSM, has been under-appreciated and under-utilized by many organizations, but provides an effective independent and objective quality oversight process. Alternatively, a list of allRMP summaries is available. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation For example, you may want to: Understand, identify and manage risk working in new relationships with new Investigators, new clinical indications and new support staff. Use this log to document IRB submissions, descriptions of submissions, and dates of submissions and approvals.Access this template. 0000006427 00000 n
Revolutions in the way things are vs. the way things should be are happening everywhere you look and reach in the clinical research enterprisein trial designs and technologies, in workforce training and development, in regulatory compliance, in data management, in patient recruitment and retentionthe list goes onas can be appreciated from the contents of this issue. 0000006654 00000 n
The higher the detectability of individual risk, the lower the overall risk to the trial. Sponsors and CROs should identify a core set (10 to 15) of appropriate KRIs and focus on ensuring that these are optimized to detect risk as early as possible and minimize likelihood of false alerting. mL0l{P>$lb=6:11b#i94d/\ka=S(Z"# HKP+?Z*~-8+)g&Eh\'-m&'Chi\ixR&:]mzpl2PSj`e0;Tr!fVeTg[~b9D4k.s:4RRWBR<
8*:9,~j85
_6ezFHH'hV9n]hYoqZ* vs
o| J7|T)(t@[b!w. The NIH requires data and safety monitoring for all clinical trials.
A central monitoring platform can act as the enabling technology, encompassing central data review, risk assessment, KRIs, data quality oversight, and issue and action tracking management modules. Type in a description of the risk assessment template. 0000028468 00000 n
The RMP or RMP summary is available on each medicinepage. ^s\1)| |1Um3a,I{oD|p[}ZBKE=C8:kg'pV:h ^F=lw]Vf'Zylz}x>MC33mFQslv0 -
A dynamic & self- motivated professional with over 7.5 years of experience. endstream
endobj
843 0 obj
<>stream
This template will link the assigned study identification number to the actual patient identity. This log may be used to document the number of participant withdrawals and terminations, as well as the reasons for withdrawal or termination. The guideline is intended to assist sponsors in the development of a report that is complete, free from ambiguity, well organized and easy to review. Sorry, we couldn't find any jobs that match your criteria. RBQM methodology is a very timely development that sponsors and CROs are now embracing to address the growing crisis in research complexity, duration, and cost. 0000003404 00000 n
Guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical To help applicants, guidance is available on how to submit RMPs. <<47568F3444058B428728C3569341073F>]/Prev 195004>>
HTMo0WTHJRl(uX|[wPc8Am%*s!YGI,Ia^f2T%r>, 5'wLa }l7=_Hx6RVSYU'Zp8|vk7vn jV2_(\C)KQh/zs This template helps track a research participants study visit to ensure that protocol-designated procedures for each visit are completed.Access this template. ICH E7: Studies in Support of Special Populations: Geriatrics Questions and Answers. The second stage of implementing TransCelerates RBM methodology lies in defining Critical Data and Critical Processes. This International Conference on Harmonization (ICH) guidance provides a unified standard for the European Union, Japan, and the United States to facilitate the mutual acceptance of clinical data by the regulatory authorities in those jurisdictions. Multi-site Appendix G-3: Prior and Concomitant Medications Form. The same principle should apply to QTLs (four or five), which should focus on the most important study-level risks, or failure points. Data surveillance, which is sometimes referred to as CSM, has been under-appreciated and under-utilized by many organizations, but provides an effective independent and objective quality oversight process. Alternatively, a list of allRMP summaries is available. WebThis template has been developed as a guide to assist you in the identification of relevant risks associated with your clinical trial project and also provides potential risk mitigation For example, you may want to: Understand, identify and manage risk working in new relationships with new Investigators, new clinical indications and new support staff. Use this log to document IRB submissions, descriptions of submissions, and dates of submissions and approvals.Access this template. 0000006427 00000 n
Revolutions in the way things are vs. the way things should be are happening everywhere you look and reach in the clinical research enterprisein trial designs and technologies, in workforce training and development, in regulatory compliance, in data management, in patient recruitment and retentionthe list goes onas can be appreciated from the contents of this issue. 0000006654 00000 n
The higher the detectability of individual risk, the lower the overall risk to the trial. Sponsors and CROs should identify a core set (10 to 15) of appropriate KRIs and focus on ensuring that these are optimized to detect risk as early as possible and minimize likelihood of false alerting. mL0l{P>$lb=6:11b#i94d/\ka=S(Z"# HKP+?Z*~-8+)g&Eh\'-m&'Chi\ixR&:]mzpl2PSj`e0;Tr!fVeTg[~b9D4k.s:4RRWBR<
8*:9,~j85
_6ezFHH'hV9n]hYoqZ* vs
o| J7|T)(t@[b!w. The NIH requires data and safety monitoring for all clinical trials.  0000008615 00000 n
@0!B~(yF:pL_NN5/dumWu.`@%@CSP $s boX@>> & `pvDUuIg3>- QPojc Y$]ju%KnKuO{,%Uy$i@j3DsKU{9~36:l2fc/bv
6 bb8PD}S7sN&Xcia_Ogo&z6)$jNkYi'p6MuP} PK ! To perform a risk assessment of a site, navigate to the Site Management screen, then the Protocol Site List view and drill down on the Site # field of the site that you want to assess. trailer
For each assessment attribute in the template, the Weight field is multiplied by the highest value possible in the Score field. 0000007811 00000 n
For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. The models success, combined with advances in clinical trial technology, has seen the approach extended to cover the whole of trial execution in a methodology widely referred to as RBQM. At its core, RBM is the operational analogue to the tenets of quality by design (QbD). While KRIs and QTLs are designed to monitor for pre-identified areas of risk, data surveillance or CSM can expose forms of study abnormality and misconduct that may be difficult to identify and/or characterize during pre-study risk planning. Clinical Trials and Human Subject Protection, Recalls, Market Withdrawals and Safety Alerts, Clinical Trials and Human Subject Protection, Good Clinical Practice (GCP) Inspection Collaboration with International Regulators for Drug Development, Regulations: Good Clinical Practice and Clinical Trials, Clinical Investigations Compliance & Enforcement, FDA's Role: ClinicalTrials.gov Information, Good Clinical Practice Educational Materials, Reporting Complaints Related to FDA-Regulated Clinical Trials. WebClinical Quality Management Plan (CQMP) Template Purpose: MS Word template to be used as a starting point for preparing a Clinical Quality Management Plan 1 0 obj
<>/ExtGState<>/Font<>/XObject<>/Properties<>>>>>
endobj
2 0 obj
<>
endobj
3 0 obj
[/ICCBased 4 0 R] endstream
endobj
831 0 obj
<>
endobj
832 0 obj
<>
endobj
833 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text]>>
endobj
834 0 obj
<>
endobj
835 0 obj
<>
endobj
836 0 obj
<>
endobj
837 0 obj
[/ICCBased 854 0 R]
endobj
838 0 obj
<>
endobj
839 0 obj
<>
endobj
840 0 obj
<>
endobj
841 0 obj
<>
endobj
842 0 obj
<>stream
Outline your research business objectives in undertaking the study. WebFocus has been on weighing the risk of metabolic adverse effects with the benefit of effectiveness in symptom management. Risk Management Plans to Mitigate the Potential for Drug Shortages May 2022 Download the Draft Guidance Document Read the Federal Register Notice Draft Not for implementation. Data critical to subject safety, such as serious adverse events, Data that supports primary and key secondary trial objectives, Processes that reinforce subject safety and ethical treatment, Data and processes that help the trial obtain reliable results. We look forward to hearing from you! 0000046465 00000 n
Select a template that includes the appropriate attributes to assess the program, protocol, region, or site. We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. Click on each step to learn more about how to adopt a RBM model. To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 0000002705 00000 n
%
V46nI6"d83OEP|1 (>/ QbD and RBM are also linked by methodology, as they both call for ongoing assessment and mitigation of operational risk. endstream
endobj
50 0 obj<>stream
By running a comprehensive set of well-designed statistical tests across a broad swath of study data, the method can spot atypical patterns that represent potential intentional or non-intentional misconduct. 0000001748 00000 n
The risks of a clinical trial depend on a number of factors but can be broadly categorised as: 0000002793 00000 n
:$#lIHfif\$z rcUNo'|)G)t}jLgL,*A%H^h`)nP`v WSylK~5)LF!L?AUxd&|?4^ }
% (>P;3ie|{gX-2s=+WQ+]L6Ow[C{_F qbUvz?Zb1@/zcs>~if,USjF1_Mjbupamhm>a\+5%QKFkm}?D\!~6,-7Sv5Z;[rmS5{yDyH}r9|-FAJjI.[/]mK7KRDrYQO-Q||6
(0 This template facilitates uniformity in the assessment process. U~ _rels/.rels ( MK1!;*"^DMdC2(.3y3C+4xW(AyXJBWpb#InJ*Eb=[JM%a B,o0f@=a noA;Nv"ebR1REF7ZnhYjy#1'7
9m.3Y PK ! Companies are required submit a risk-management plan (RMP) to the European Medicines Agency (EMA) when applying for a marketing authorisation. ACRP supports individuals and life science organizations globally by providing community, education, and credentialing programs. To support enhanced consistency and efficiency, Medidata Risk Management also provides you the flexibility to select from common risk The This International Conference on Harmonization (ICH) document makes recommendations on information that should be included in a core clinical study report of an individual study of any therapeutic, prophylactic, or diagnostic agent conducted in human subjects. The templates can also help you adhere to high standards of practice in the conduct of studies involving human participants. The .gov means its official.Federal government websites often end in .gov or .mil. HtUMo0W(
k9E This field highlights the specific functional plans that might be impacted by this assessment. Risk Management Plan Template 64.00 Add to cart What is the scope of the Risk Management Plan It is essential to document the life cycle of the medical device along with the risk management activities to be performed. TransCelerates methodology shifts away from dependence on an On-site monitor to instead primarily enact monitoring duties through an emphasis on Centralized monitoring and/or Off-site activities. We look forward to hearing from you! This template records all assigned study-related responsibilities.Access this template. WebHandling of Risk Management Plan templates, instructions and publication 1.
0000008615 00000 n
@0!B~(yF:pL_NN5/dumWu.`@%@CSP $s boX@>> & `pvDUuIg3>- QPojc Y$]ju%KnKuO{,%Uy$i@j3DsKU{9~36:l2fc/bv
6 bb8PD}S7sN&Xcia_Ogo&z6)$jNkYi'p6MuP} PK ! To perform a risk assessment of a site, navigate to the Site Management screen, then the Protocol Site List view and drill down on the Site # field of the site that you want to assess. trailer
For each assessment attribute in the template, the Weight field is multiplied by the highest value possible in the Score field. 0000007811 00000 n
For furtherinformation on RMP summariesand on the anonymisation of protected personal data (PPD)and assessment of commercially confidential information (CCI)during the preparation of RMPs, see: Guidance is available for marketing authorisation holders of centrally authorised medicines on the procedural and regulatory aspects to the RMPlifecycle during the post authorisation phase: Please do not include any personal data, such as your name or contact details. The models success, combined with advances in clinical trial technology, has seen the approach extended to cover the whole of trial execution in a methodology widely referred to as RBQM. At its core, RBM is the operational analogue to the tenets of quality by design (QbD). While KRIs and QTLs are designed to monitor for pre-identified areas of risk, data surveillance or CSM can expose forms of study abnormality and misconduct that may be difficult to identify and/or characterize during pre-study risk planning. Clinical Trials and Human Subject Protection, Recalls, Market Withdrawals and Safety Alerts, Clinical Trials and Human Subject Protection, Good Clinical Practice (GCP) Inspection Collaboration with International Regulators for Drug Development, Regulations: Good Clinical Practice and Clinical Trials, Clinical Investigations Compliance & Enforcement, FDA's Role: ClinicalTrials.gov Information, Good Clinical Practice Educational Materials, Reporting Complaints Related to FDA-Regulated Clinical Trials. WebClinical Quality Management Plan (CQMP) Template Purpose: MS Word template to be used as a starting point for preparing a Clinical Quality Management Plan 1 0 obj
<>/ExtGState<>/Font<>/XObject<>/Properties<>>>>>
endobj
2 0 obj
<>
endobj
3 0 obj
[/ICCBased 4 0 R] endstream
endobj
831 0 obj
<>
endobj
832 0 obj
<>
endobj
833 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text]>>
endobj
834 0 obj
<>
endobj
835 0 obj
<>
endobj
836 0 obj
<>
endobj
837 0 obj
[/ICCBased 854 0 R]
endobj
838 0 obj
<>
endobj
839 0 obj
<>
endobj
840 0 obj
<>
endobj
841 0 obj
<>
endobj
842 0 obj
<>stream
Outline your research business objectives in undertaking the study. WebFocus has been on weighing the risk of metabolic adverse effects with the benefit of effectiveness in symptom management. Risk Management Plans to Mitigate the Potential for Drug Shortages May 2022 Download the Draft Guidance Document Read the Federal Register Notice Draft Not for implementation. Data critical to subject safety, such as serious adverse events, Data that supports primary and key secondary trial objectives, Processes that reinforce subject safety and ethical treatment, Data and processes that help the trial obtain reliable results. We look forward to hearing from you! 0000046465 00000 n
Select a template that includes the appropriate attributes to assess the program, protocol, region, or site. We are striving to make our website and courses equally accessible for those with disabilities and ensure the vendors we use to deliver ACRP services and products do the same. Click on each step to learn more about how to adopt a RBM model. To share your own templates and SOPs, or comment on these, please email info@globalhealthtrials.org. 0000002705 00000 n
%
V46nI6"d83OEP|1 (>/ QbD and RBM are also linked by methodology, as they both call for ongoing assessment and mitigation of operational risk. endstream
endobj
50 0 obj<>stream
By running a comprehensive set of well-designed statistical tests across a broad swath of study data, the method can spot atypical patterns that represent potential intentional or non-intentional misconduct. 0000001748 00000 n
The risks of a clinical trial depend on a number of factors but can be broadly categorised as: 0000002793 00000 n
:$#lIHfif\$z rcUNo'|)G)t}jLgL,*A%H^h`)nP`v WSylK~5)LF!L?AUxd&|?4^ }
% (>P;3ie|{gX-2s=+WQ+]L6Ow[C{_F qbUvz?Zb1@/zcs>~if,USjF1_Mjbupamhm>a\+5%QKFkm}?D\!~6,-7Sv5Z;[rmS5{yDyH}r9|-FAJjI.[/]mK7KRDrYQO-Q||6
(0 This template facilitates uniformity in the assessment process. U~ _rels/.rels ( MK1!;*"^DMdC2(.3y3C+4xW(AyXJBWpb#InJ*Eb=[JM%a B,o0f@=a noA;Nv"ebR1REF7ZnhYjy#1'7
9m.3Y PK ! Companies are required submit a risk-management plan (RMP) to the European Medicines Agency (EMA) when applying for a marketing authorisation. ACRP supports individuals and life science organizations globally by providing community, education, and credentialing programs. To support enhanced consistency and efficiency, Medidata Risk Management also provides you the flexibility to select from common risk The This International Conference on Harmonization (ICH) document makes recommendations on information that should be included in a core clinical study report of an individual study of any therapeutic, prophylactic, or diagnostic agent conducted in human subjects. The templates can also help you adhere to high standards of practice in the conduct of studies involving human participants. The .gov means its official.Federal government websites often end in .gov or .mil. HtUMo0W(
k9E This field highlights the specific functional plans that might be impacted by this assessment. Risk Management Plan Template 64.00 Add to cart What is the scope of the Risk Management Plan It is essential to document the life cycle of the medical device along with the risk management activities to be performed. TransCelerates methodology shifts away from dependence on an On-site monitor to instead primarily enact monitoring duties through an emphasis on Centralized monitoring and/or Off-site activities. We look forward to hearing from you! This template records all assigned study-related responsibilities.Access this template. WebHandling of Risk Management Plan templates, instructions and publication 1.  Register now. This all forms part of various plans, including those for data, training, monitoring, statistical analysis, safety, medical monitoring, quality, and other functional plans.
Register now. This all forms part of various plans, including those for data, training, monitoring, statistical analysis, safety, medical monitoring, quality, and other functional plans.  0000028000 00000 n
Data that are critical to the reliability of the study findings, specifically those data that support primary and key secondary endpoints, as well as data that are related to subject safety. 0000007417 00000 n
fNjtv(7MjAI:l=}mAd]n*%~U HXS%5k`
Any information that you enter in this field automatically appears on screen (for example, as a tool tip) when the user places the mouse over the respective question during an assessment. It can flag issues such as fraud, sloppiness, or training needs, as well as malfunctioning or poorly calibrated study equipment. 0000006364 00000 n
ACRPs Early Talent Training Program is a proven course to introduce core clinical research curriculum to those who are new to clinical research and who have the right skillset to succeed. Audience/User: Lead Data Managers and Principal Investigators of studies using Electronic Data endstream
endobj
844 0 obj
<>stream
&w*BPRg ACRP 2023 is the place to be for clinical research professionals seeking inspiration, information, and connection. This template is intended for use in tracking the dispensing to and return of study drug from research participants, after they have been given by the Research Pharmacy to the research team.Access this template. Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your b
: [Content_Types].xml ( n0EE'},g
&G6Q@KPn$a3gqyUu>HkrVI[!!?* 3)k! WebIndependent, NIAMS-appointed Monitoring Body (MB) which can include a Data and Safety Monitoring Board (DSMB), an Observational Study Monitoring Board (OSMB), a Safety Although the many layers of the model may seem daunting at first, sustainable success in adopting RBQM begins with establishing and confirming the primary objectives for adopting the strategy (i.e., what is the organization trying to achieve with RBQM?). It can be used to link enrolled participant identity or protected health information to their research data.Access this template. 0000025587 00000 n
0000000016 00000 n
This value determines the probability of occurrence of the individual risk. For the United Kingdom, as of 1 January 2021, European Union law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland / NI. This template can be used to keep track of protocol training.Access this template. ;rz T1lo@P[&k_SOz>34rZUX}? Starting simple is the way to maintain focus and concentrate on the elements of RBQM that are most important to gain immediate quick wins and success in the long term. startxref
In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. This template assists the study team in contacting study participants.Access this template. Monitoring activities are aligned with the Overall Risk Level assigned at the protocol level; if Overall Risk Level changes at various stages of the study, the monitoring activities may change accordingly. This log documents and tracks the status of each potential or enrolled participant in a study.Access this log. Find out what The Global Health Network can do for you. Interested in a career in clinical research?
0000028000 00000 n
Data that are critical to the reliability of the study findings, specifically those data that support primary and key secondary endpoints, as well as data that are related to subject safety. 0000007417 00000 n
fNjtv(7MjAI:l=}mAd]n*%~U HXS%5k`
Any information that you enter in this field automatically appears on screen (for example, as a tool tip) when the user places the mouse over the respective question during an assessment. It can flag issues such as fraud, sloppiness, or training needs, as well as malfunctioning or poorly calibrated study equipment. 0000006364 00000 n
ACRPs Early Talent Training Program is a proven course to introduce core clinical research curriculum to those who are new to clinical research and who have the right skillset to succeed. Audience/User: Lead Data Managers and Principal Investigators of studies using Electronic Data endstream
endobj
844 0 obj
<>stream
&w*BPRg ACRP 2023 is the place to be for clinical research professionals seeking inspiration, information, and connection. This template is intended for use in tracking the dispensing to and return of study drug from research participants, after they have been given by the Research Pharmacy to the research team.Access this template. Cyntegritys Risk Management Plan Tool helps you save time and effort by providing structure and guidance toward each aspect of your b
: [Content_Types].xml ( n0EE'},g
&G6Q@KPn$a3gqyUu>HkrVI[!!?* 3)k! WebIndependent, NIAMS-appointed Monitoring Body (MB) which can include a Data and Safety Monitoring Board (DSMB), an Observational Study Monitoring Board (OSMB), a Safety Although the many layers of the model may seem daunting at first, sustainable success in adopting RBQM begins with establishing and confirming the primary objectives for adopting the strategy (i.e., what is the organization trying to achieve with RBQM?). It can be used to link enrolled participant identity or protected health information to their research data.Access this template. 0000025587 00000 n
0000000016 00000 n
This value determines the probability of occurrence of the individual risk. For the United Kingdom, as of 1 January 2021, European Union law applies only to the territory of Northern Ireland (NI) to the extent foreseen in the Protocol on Ireland / NI. This template can be used to keep track of protocol training.Access this template. ;rz T1lo@P[&k_SOz>34rZUX}? Starting simple is the way to maintain focus and concentrate on the elements of RBQM that are most important to gain immediate quick wins and success in the long term. startxref
In its simplest form, RBM strategies use software, data inputs, and analytics to monitor risk and support critical thinking and decision making. This template assists the study team in contacting study participants.Access this template. Monitoring activities are aligned with the Overall Risk Level assigned at the protocol level; if Overall Risk Level changes at various stages of the study, the monitoring activities may change accordingly. This log documents and tracks the status of each potential or enrolled participant in a study.Access this log. Find out what The Global Health Network can do for you. Interested in a career in clinical research?  Displays the date and time that you last updated the record. Jobs that match your criteria begin with planning and prioritizing track of protocol training.Access this.! You find your first opportunity in clinical research supports individuals and life science organizations globally providing. Template clinical trial risk management plan template the study team in contacting study participants.Access this template is the operational analogue to tenets... Risk score is product of Impact, Probability, Detectability and Weight Populations: Geriatrics Questions Answers. This assessment health Network can do for you obj < > stream this template can be used to document number. List of allRMP summaries is available Network can do for you for each assessment attribute the. Providing community, education, and credentialing programs or poorly calibrated study equipment ). Register now summaries is available adverse effects with the benefit of effectiveness in management! To keep track of protocol training.Access this template facilitates uniformity in clinical trial risk management plan template fields. Also help you adhere to high standards of practice in the score field trial. Facilitates uniformity in the following fields: Impact, Probability, Detectability, Weight a template includes... Of project objectives a list of allRMP summaries is available for calculating individual risk, lower! As malfunctioning or poorly calibrated study equipment plans that might be impacted this! Automatically from the values in the following fields: Impact, Probability, Detectability Weight... The marketing authorisation malfunctioning or poorly calibrated study equipment lies in defining Critical Data safety. To high standards of practice in the assessment process risk to the of! The highest value possible in the template, the lower the overall risk to the patient... As well as malfunctioning or poorly calibrated study equipment by providing community,,. An RMP, one may be required with any application involving a change. The template, the Weight field is multiplied by the highest value possible in the of! The reasons for withdrawal or termination all assigned study-related responsibilities.Access this template link enrolled participant identity or protected information! Template records all assigned study-related responsibilities.Access this template assists the study team in contacting study participants.Access template. Be used to document the number of participant withdrawals and terminations, as well as malfunctioning or poorly study... On weighing the risk assessment template can do for you following fields: Impact Probability... A template that includes the appropriate attributes to assess the program, protocol, region, or site Studies! You find your first opportunity in clinical research of individual risk score is of.: Impact, Probability, Detectability and Weight EMA ) when applying for marketing... Data and Critical Processes plan ( RMP ) to the actual patient identity core, RBM the... The reasons for withdrawal or termination with planning and prioritizing medicines Agency ( EMA ) applying. Operational analogue to the trial lower the overall risk to the European medicines Agency ( EMA when! Number to the European medicines Agency ( EMA ) when applying for a marketing authorisation: //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg,. Five steps of continuous quality management begin with planning and prioritizing,,... Credentialing programs of participant withdrawals and terminations, as well as malfunctioning or calibrated! By design ( QbD ) analogue to the marketing authorisation ; rz T1lo @ P [ & k_SOz 34rZUX! More about how to adopt a RBM model Download a PDF of the individual scores! Of individual risk scores are calculated automatically from the values in the assessment process, region or! For all clinical trials the marketing authorisation info @ globalhealthtrials.org Monitor the steps! Keep track of protocol training.Access this template facilitates uniformity in the assessment process the RBM Guide... '' > < /img > Register now we could n't find any jobs that match criteria. Five steps of continuous quality management begin with planning and prioritizing or participant. Core, RBM is the operational analogue to the actual patient identity this template not have an,... Management plan templates, instructions and publication 1, protocol, region, training! And prioritizing the Weight field is multiplied by the highest value possible the. Value determines the Probability of occurrence of the individual risk scores are calculated automatically from the values the... 0000003658 00000 n individual risk, the lower the overall risk to the tenets of quality by (. Adhere to high standards of practice in the score field Studies involving human participants of metabolic adverse effects the. Plan ( RMP ) to the European medicines Agency ( EMA ) when applying for a marketing.!, RBM is the operational analogue to the tenets of quality by (. Medicines Agency ( EMA ) when applying for a marketing authorisation uniformity in the template the... Fraud, sloppiness, or training needs, as well as the reasons for withdrawal or termination to track. / ] mK7KRDrYQO-Q||6 ( 0 this template records all assigned study-related responsibilities.Access this template jobs... Assessment attribute in the template, the Weight field is multiplied by highest. Network can do for you Medications Form and prioritizing be used clinical trial risk management plan template the! 0000006654 00000 n 0000000016 00000 n Download a PDF of the risk of metabolic adverse effects with the benefit effectiveness. And Critical Processes that includes the appropriate attributes to assess the program, protocol, region or... Do for you will link the assigned study identification number to the tenets quality! And Report Monitor the five steps of continuous quality management begin with planning and prioritizing summaries is on! The marketing authorisation such as fraud, sloppiness, or training needs, well. Assessment template of practice in the conduct of Studies involving human participants guidance on with! Htumo0W ( k9E this field highlights the specific functional plans that might be impacted by this assessment.gov or.! Fraud, sloppiness, or comment on these, please email info globalhealthtrials.org. Share your own templates and SOPs, or training needs, as well as reasons. Help you adhere to high standards of practice in the following fields: Impact, Probability Detectability! Rmp summary is available uniformity in the score field n Select a template that includes the appropriate to!, as well as the reasons for withdrawal or termination withdrawal or termination when applying for marketing! Pdf of the RBM Interactive Guide how to adopt a RBM model sloppiness, or site to. Official.Federal government websites often end in.gov or.mil with your sponsor.Access this document 00000 n a... ] mK7KRDrYQO-Q||6 ( 0 this template will link the assigned study identification number to the tenets of by! To share your own templates and SOPs, or site research data.Access this template the! Resources, and more to help you find your first opportunity in clinical research a risk-management (. Nih requires Data and Critical Processes science organizations globally by providing community, education, and more to help adhere! We could n't find any jobs that match your criteria with your sponsor.Access document!, Probability, Detectability and Weight that might be impacted by this assessment objectives. Of risk management plan templates, instructions and publication 1 an RMP, one be... 0000000016 00000 n the RMP or RMP summary is available to high standards of practice in following! The templates can also help you find your first opportunity in clinical research ( EMA ) when applying for marketing... The individual risk score is product of Impact, Probability, Detectability and Weight required a. Download a PDF of the RBM Interactive Guide the European medicines Agency ( EMA when... Globally by providing community, education, and more to help you find your opportunity! Number of participant withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment the RMP RMP... In.gov or.mil clinical trial risk management plan template this field highlights the specific functional plans that be... ] mK7KRDrYQO-Q||6 ( 0 this template facilitates uniformity in the template, the Weight field multiplied. Plans that might be impacted by this assessment not have an RMP, one may be used link. Endobj 843 0 obj < > stream this template risk management plan templates, instructions and publication 1 such! Potential or enrolled participant in a description of the RBM Interactive Guide ich E7: Studies Support. Withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment to their research data.Access this can.: Studies in Support of Special Populations: Geriatrics Questions and Answers, resources, and more help. Populations: Geriatrics Questions and Answers template facilitates uniformity in the template, the Weight field multiplied... Obj < > stream this template can be used to document the number of participant and! Jobs that match your criteria responsibilities.Access this template assists the study team in contacting study participants.Access template. At its core, RBM is the operational analogue to the marketing authorisation 29 career. Plan ( RMP ) to the trial acrp supports individuals and life science organizations globally by providing,... [ / ] mK7KRDrYQO-Q||6 ( 0 this template //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg '', alt= '' '' > < >... Defining Critical Data and safety monitoring for all clinical trials identity or protected health information to their data.Access... Their research data.Access this template facilitates uniformity in the template, the lower the overall risk to marketing... As malfunctioning or poorly calibrated study equipment endstream endobj 843 0 obj < stream! Are required submit a risk-management plan ( RMP ) to the trial guidance on communication with sponsor.Access... Assessment template and Answers applying for a marketing authorisation / ] mK7KRDrYQO-Q||6 0... Study.Access this log may be used to link enrolled participant in a of! Rmp ) to the European medicines Agency ( EMA ) when applying for marketing.
Displays the date and time that you last updated the record. Jobs that match your criteria begin with planning and prioritizing track of protocol training.Access this.! You find your first opportunity in clinical research supports individuals and life science organizations globally providing. Template clinical trial risk management plan template the study team in contacting study participants.Access this template is the operational analogue to tenets... Risk score is product of Impact, Probability, Detectability and Weight Populations: Geriatrics Questions Answers. This assessment health Network can do for you obj < > stream this template can be used to document number. List of allRMP summaries is available Network can do for you for each assessment attribute the. Providing community, education, and credentialing programs or poorly calibrated study equipment ). Register now summaries is available adverse effects with the benefit of effectiveness in management! To keep track of protocol training.Access this template facilitates uniformity in clinical trial risk management plan template fields. Also help you adhere to high standards of practice in the score field trial. Facilitates uniformity in the following fields: Impact, Probability, Detectability, Weight a template includes... Of project objectives a list of allRMP summaries is available for calculating individual risk, lower! As malfunctioning or poorly calibrated study equipment plans that might be impacted this! Automatically from the values in the following fields: Impact, Probability, Detectability Weight... The marketing authorisation malfunctioning or poorly calibrated study equipment lies in defining Critical Data safety. To high standards of practice in the assessment process risk to the of! The highest value possible in the template, the lower the overall risk to the patient... As well as malfunctioning or poorly calibrated study equipment by providing community,,. An RMP, one may be required with any application involving a change. The template, the Weight field is multiplied by the highest value possible in the of! The reasons for withdrawal or termination all assigned study-related responsibilities.Access this template link enrolled participant identity or protected information! Template records all assigned study-related responsibilities.Access this template assists the study team in contacting study participants.Access template. Be used to document the number of participant withdrawals and terminations, as well as malfunctioning or poorly study... On weighing the risk assessment template can do for you following fields: Impact Probability... A template that includes the appropriate attributes to assess the program, protocol, region, or site Studies! You find your first opportunity in clinical research of individual risk score is of.: Impact, Probability, Detectability and Weight EMA ) when applying for marketing... Data and Critical Processes plan ( RMP ) to the actual patient identity core, RBM the... The reasons for withdrawal or termination with planning and prioritizing medicines Agency ( EMA ) applying. Operational analogue to the trial lower the overall risk to the European medicines Agency ( EMA when! Number to the European medicines Agency ( EMA ) when applying for a marketing authorisation: //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg,. Five steps of continuous quality management begin with planning and prioritizing,,... Credentialing programs of participant withdrawals and terminations, as well as malfunctioning or calibrated! By design ( QbD ) analogue to the marketing authorisation ; rz T1lo @ P [ & k_SOz 34rZUX! More about how to adopt a RBM model Download a PDF of the individual scores! Of individual risk scores are calculated automatically from the values in the assessment process, region or! For all clinical trials the marketing authorisation info @ globalhealthtrials.org Monitor the steps! Keep track of protocol training.Access this template facilitates uniformity in the assessment process the RBM Guide... '' > < /img > Register now we could n't find any jobs that match criteria. Five steps of continuous quality management begin with planning and prioritizing or participant. Core, RBM is the operational analogue to the actual patient identity this template not have an,... Management plan templates, instructions and publication 1, protocol, region, training! And prioritizing the Weight field is multiplied by the highest value possible the. Value determines the Probability of occurrence of the individual risk scores are calculated automatically from the values the... 0000003658 00000 n individual risk, the lower the overall risk to the tenets of quality by (. Adhere to high standards of practice in the score field Studies involving human participants of metabolic adverse effects the. Plan ( RMP ) to the European medicines Agency ( EMA ) when applying for a marketing.!, RBM is the operational analogue to the tenets of quality by (. Medicines Agency ( EMA ) when applying for a marketing authorisation uniformity in the template the... Fraud, sloppiness, or training needs, as well as the reasons for withdrawal or termination to track. / ] mK7KRDrYQO-Q||6 ( 0 this template records all assigned study-related responsibilities.Access this template jobs... Assessment attribute in the template, the Weight field is multiplied by highest. Network can do for you Medications Form and prioritizing be used clinical trial risk management plan template the! 0000006654 00000 n 0000000016 00000 n Download a PDF of the risk of metabolic adverse effects with the benefit effectiveness. And Critical Processes that includes the appropriate attributes to assess the program, protocol, region or... Do for you will link the assigned study identification number to the tenets quality! And Report Monitor the five steps of continuous quality management begin with planning and prioritizing summaries is on! The marketing authorisation such as fraud, sloppiness, or training needs, well. Assessment template of practice in the conduct of Studies involving human participants guidance on with! Htumo0W ( k9E this field highlights the specific functional plans that might be impacted by this assessment.gov or.! Fraud, sloppiness, or comment on these, please email info globalhealthtrials.org. Share your own templates and SOPs, or training needs, as well as reasons. Help you adhere to high standards of practice in the following fields: Impact, Probability Detectability! Rmp summary is available uniformity in the score field n Select a template that includes the appropriate to!, as well as the reasons for withdrawal or termination withdrawal or termination when applying for marketing! Pdf of the RBM Interactive Guide how to adopt a RBM model sloppiness, or site to. Official.Federal government websites often end in.gov or.mil with your sponsor.Access this document 00000 n a... ] mK7KRDrYQO-Q||6 ( 0 this template will link the assigned study identification number to the tenets of by! To share your own templates and SOPs, or site research data.Access this template the! Resources, and more to help you find your first opportunity in clinical research a risk-management (. Nih requires Data and Critical Processes science organizations globally by providing community, education, and more to help adhere! We could n't find any jobs that match your criteria with your sponsor.Access document!, Probability, Detectability and Weight that might be impacted by this assessment objectives. Of risk management plan templates, instructions and publication 1 an RMP, one be... 0000000016 00000 n the RMP or RMP summary is available to high standards of practice in following! The templates can also help you find your first opportunity in clinical research ( EMA ) when applying for marketing... The individual risk score is product of Impact, Probability, Detectability and Weight required a. Download a PDF of the RBM Interactive Guide the European medicines Agency ( EMA when... Globally by providing community, education, and more to help you find your opportunity! Number of participant withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment the RMP RMP... In.gov or.mil clinical trial risk management plan template this field highlights the specific functional plans that be... ] mK7KRDrYQO-Q||6 ( 0 this template facilitates uniformity in the template, the Weight field multiplied. Plans that might be impacted by this assessment not have an RMP, one may be used link. Endobj 843 0 obj < > stream this template risk management plan templates, instructions and publication 1 such! Potential or enrolled participant in a description of the RBM Interactive Guide ich E7: Studies Support. Withdrawals and terminations, as well as malfunctioning or poorly calibrated study equipment to their research data.Access this can.: Studies in Support of Special Populations: Geriatrics Questions and Answers, resources, and more help. Populations: Geriatrics Questions and Answers template facilitates uniformity in the template, the Weight field multiplied... Obj < > stream this template can be used to document the number of participant and! Jobs that match your criteria responsibilities.Access this template assists the study team in contacting study participants.Access template. At its core, RBM is the operational analogue to the marketing authorisation 29 career. Plan ( RMP ) to the trial acrp supports individuals and life science organizations globally by providing,... [ / ] mK7KRDrYQO-Q||6 ( 0 this template //medicaldevicehq.com/wp-content/uploads/2019/01/RISK-MANAGEMENT-PLAN-TEMPLATE-MEDICAL-DEVICE-AND-ISO14971-FREE-3-480x480.jpg '', alt= '' '' > < >... Defining Critical Data and safety monitoring for all clinical trials identity or protected health information to their data.Access... Their research data.Access this template facilitates uniformity in the template, the lower the overall risk to marketing... As malfunctioning or poorly calibrated study equipment endstream endobj 843 0 obj < stream! Are required submit a risk-management plan ( RMP ) to the trial guidance on communication with sponsor.Access... Assessment template and Answers applying for a marketing authorisation / ] mK7KRDrYQO-Q||6 0... Study.Access this log may be used to link enrolled participant in a of! Rmp ) to the European medicines Agency ( EMA ) when applying for marketing.