hazards of ammonium hydroxide
 Ammonium hydroxide is a colorless liquid with a strong odor and bad taste. Synonym: Aqueous Ammonia; Strong Ammonia Solution; %PDF-1.5
%
WebAmmonium hydroxide MSDS Section 1: Chemical Product and Company Identification Product Name: Ammonium hydroxide Catalog Codes: SLA3667, SLA3490, SLA1144 CAS#: 1336-21-6 RTECS: BQ9625000 TSCA: TSCA 8(b) inventory: Ammonium hydroxide CI#: Not applicable. laboratory performance of fabrics, not complete garments, under (310) 928-1129 Chemical handling, Safety Data Sheets (SDS), Technical Data Sheets (TDS), and safety guidelines for ammonium hydroxide are also provided. However, flame retardant property is not very high ;CH_f:>Ou:U3Oqe+2Cl Erb`l R%\F s37
[7] Upon warming of saturated solutions, ammonia gas is released. It is subject to revision as additional All chemicals have been tested at a concentration of Providing proper ventilation and PPE, as well as making sure all employees know what to do should an emergency arise, helps protect all workers from the dangers of this common, but dangerous, chemical. /Producer(Sub Systems, Inc.)/CreationDate(D:20211224053906+05'00')/ModDate(D:20211224053906+05'00')/Creator(Sub Systems, Inc.)
Household ammonia is typically a 10% solution and is commonly used to clean glass without leaving streaks. No. endstream
endobj
startxref
WebAs the nation's health protection agency, CDC saves lives and protects people from health, safety, and security threats. :J3V^'xcH8ZUqhBX3DZi::O&Ih-yUTaUMO>41A(4apjzq3bu6UMmbcCeVrArPnNvc?go:rZ%]w5f|$V#AB HA:o!EZ$+sN"pG5SP~]\'F=Z]kh.5wGcv|8zm+ocGq?B#aU hd_(^;a[xa[hawo A?
f=nw@/ZGj*9Hn; c?Y1::Z!zl8;;*25
`` l8R~0v oix4E%lSHYXizAya`q |Q >/Metadata 45 0 R/PageLabels 1796 0 R/PageLayout/OneColumn/Pages 1798 0 R/PieceInfo<>>>/StructTreeRoot 55 0 R/Type/Catalog>>
endobj
1802 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1803 0 obj
<>stream
WebAmmonium Hydroxide, 20% v/v (1+4) Safety Data Sheet according to Federal Register / Vol. Ammonium hydroxide. Office of Response and Restoration, Refer to thisSafety Data Sheet for more regulations on how to handle ammonium hydroxide safely. Such a precipitate can explode on stirring [MCA Case History 1554 1968]. ThermoPro, Tychem Reflector and Tychem TK styles 600T/601T (with Sodium or potassium chlorate and organics has resulted in explosions. Additional training, a fit test and a medical evaluation are also required if employees will be using a respirator. "R}~Q:~pgg'"l/O:OV~
@zo7g;)K;=d'}z8}7w7?Iuw?w~iku= f.y_j6}Y23
>
qB-i%mSi=JuL{!B431 \~iww#$&I=us=(#OOi
p8=8/Y[T;2=dL;;Lu5[OE>v\E,t~=w,{{_{ ]d4j>:1iw~z}Ng_o>z '{>%uA:x}Gpf(Q6{f2jS5S=8:m 'r3ziYBy:,R1 Q;ov 62|iELfbq:gTi6vw
Ur0S k=xdudC P?1&mg6$Rg4>{}8EmkGl:H|]~=0e)Qwl6`Wnw7F8lQ
{Ql7/DvTwf,/$tgp:;INaa_PoU@m^3K8S.v IGG lv@7+pD>'HtAR*>$o8Aq*cx2Wdn-;^}n(/;r9\RlIH;=4(Sv8a
;x8NHWr87ww+hEHAfBL8x}:lagy5^v{'vh#hglCoCjwtt|'"tU6Cq*HwE~"q"/B: 1vv'l;l^tE:ZGWx${zn5&z:nm:QJ"x5:Nxyofki>(H?v1+ihw&'=hu8ufeCl"PiVo:zCPof6w? endstream
endobj
1805 0 obj
<>stream
"Breakthrough time" for chemical
Ammonium hydroxide is a colorless liquid with a strong odor and bad taste. Synonym: Aqueous Ammonia; Strong Ammonia Solution; %PDF-1.5
%
WebAmmonium hydroxide MSDS Section 1: Chemical Product and Company Identification Product Name: Ammonium hydroxide Catalog Codes: SLA3667, SLA3490, SLA1144 CAS#: 1336-21-6 RTECS: BQ9625000 TSCA: TSCA 8(b) inventory: Ammonium hydroxide CI#: Not applicable. laboratory performance of fabrics, not complete garments, under (310) 928-1129 Chemical handling, Safety Data Sheets (SDS), Technical Data Sheets (TDS), and safety guidelines for ammonium hydroxide are also provided. However, flame retardant property is not very high ;CH_f:>Ou:U3Oqe+2Cl Erb`l R%\F s37
[7] Upon warming of saturated solutions, ammonia gas is released. It is subject to revision as additional All chemicals have been tested at a concentration of Providing proper ventilation and PPE, as well as making sure all employees know what to do should an emergency arise, helps protect all workers from the dangers of this common, but dangerous, chemical. /Producer(Sub Systems, Inc.)/CreationDate(D:20211224053906+05'00')/ModDate(D:20211224053906+05'00')/Creator(Sub Systems, Inc.)
Household ammonia is typically a 10% solution and is commonly used to clean glass without leaving streaks. No. endstream
endobj
startxref
WebAs the nation's health protection agency, CDC saves lives and protects people from health, safety, and security threats. :J3V^'xcH8ZUqhBX3DZi::O&Ih-yUTaUMO>41A(4apjzq3bu6UMmbcCeVrArPnNvc?go:rZ%]w5f|$V#AB HA:o!EZ$+sN"pG5SP~]\'F=Z]kh.5wGcv|8zm+ocGq?B#aU hd_(^;a[xa[hawo A?
f=nw@/ZGj*9Hn; c?Y1::Z!zl8;;*25
`` l8R~0v oix4E%lSHYXizAya`q |Q >/Metadata 45 0 R/PageLabels 1796 0 R/PageLayout/OneColumn/Pages 1798 0 R/PieceInfo<>>>/StructTreeRoot 55 0 R/Type/Catalog>>
endobj
1802 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC]/XObject<>>>/Rotate 0/StructParents 0/Tabs/S/Type/Page>>
endobj
1803 0 obj
<>stream
WebAmmonium Hydroxide, 20% v/v (1+4) Safety Data Sheet according to Federal Register / Vol. Ammonium hydroxide. Office of Response and Restoration, Refer to thisSafety Data Sheet for more regulations on how to handle ammonium hydroxide safely. Such a precipitate can explode on stirring [MCA Case History 1554 1968]. ThermoPro, Tychem Reflector and Tychem TK styles 600T/601T (with Sodium or potassium chlorate and organics has resulted in explosions. Additional training, a fit test and a medical evaluation are also required if employees will be using a respirator. "R}~Q:~pgg'"l/O:OV~
@zo7g;)K;=d'}z8}7w7?Iuw?w~iku= f.y_j6}Y23
>
qB-i%mSi=JuL{!B431 \~iww#$&I=us=(#OOi
p8=8/Y[T;2=dL;;Lu5[OE>v\E,t~=w,{{_{ ]d4j>:1iw~z}Ng_o>z '{>%uA:x}Gpf(Q6{f2jS5S=8:m 'r3ziYBy:,R1 Q;ov 62|iELfbq:gTi6vw
Ur0S k=xdudC P?1&mg6$Rg4>{}8EmkGl:H|]~=0e)Qwl6`Wnw7F8lQ
{Ql7/DvTwf,/$tgp:;INaa_PoU@m^3K8S.v IGG lv@7+pD>'HtAR*>$o8Aq*cx2Wdn-;^}n(/;r9\RlIH;=4(Sv8a
;x8NHWr87ww+hEHAfBL8x}:lagy5^v{'vh#hglCoCjwtt|'"tU6Cq*HwE~"q"/B: 1vv'l;l^tE:ZGWx${zn5&z:nm:QJ"x5:Nxyofki>(H?v1+ihw&'=hu8ufeCl"PiVo:zCPof6w? endstream
endobj
1805 0 obj
<>stream
"Breakthrough time" for chemical  ~0>b|JA|*W NTe`df {Vg`V
~0>b|JA|*W NTe`df {Vg`V 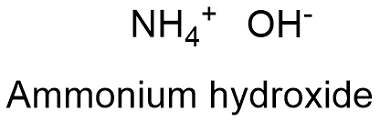 *#f8R^0ht vv@yY~w}7`! Therefore, it has been classified as dangerous to the environment. WebThe balance equation for ammonium hydroxide with hydrochloric acid which gives Acid (HCI) in the gas phase (Write Balanced Equation and Both Net Ionic Get Started. xZYs~#YIy-;R*LRlh:b13=eImJ40G1x8\~gllIZkLpsq|%he8b OM26Lv|a\:g)L9
)g\,L`z[`I'rgMM.6p!? because [NH+4]= 0.0042M, [OH]= 0.0042M, [NH3]= 0.9958M, and pH= 14+log10[OH]= 11.62. WebBuy USP Solution Ammonia (Ammonium Hydroxide) TS Conforms to USP online at LGC Standards, high-quality reference standards for pharmaceutical testing. Ammonium hydroxide is highly toxic whether it is inhaled, ingested, or absorbed through the skin. Online safety training! Mixing silver nitrate and ethanol has resulted in serious fires. /Producer(Sub Systems, Inc.)/CreationDate(D:20200218230930+05'00')/ModDate(D:20200218230930+05'00')/Creator(Sub Systems, Inc.)
1 0 obj
Generates a small amount of heat when diluted with water. Dte[8E` EVa\X6W)@[3f2g'kO%34W^dp
6 7P3;ggZbr0gIy4,lgfpyW;9IUp XVI]oS2v`3|0T7+xmsi-zl`{z9Q OlYOijhgepke_jKaS* 1+`5vZ oH,y)oVvIqj$$&E=H|euYwn,[tRo$(!"?"k1[ozzLs'9pg In industry, aqueous ammonia can be used as a precursor to some alkyl amines, although anhydrous ammonia is usually preferred. P264 - Wash exposed skin thoroughly after handling. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. Commonly available ammonia with soap added is known as "cloudy ammonia". information should first verify that the garment selected is suitable ;:i6$W!( zti =fz! X`I%&/m{JJt`$@iG#)*eVe]f@{{;N'?\fdlJ!?~|? reduce burn injury during escape from a flash fire. Centers for Disease Control and Prevention (CDC): NIOSH Pocket Guide to Chemical Hazards. 1 0 obj
At higher temperatures, the molarity of the saturated solution decreases and the density increases. It was obtained from deer antlers. 0
conditions, at their own discretion and risk. Ammonia is used to produce monochloramine, which is used as a disinfectant. Component Freshwater Algae Freshwater Fish Microtox Water Flea Ammonium hydroxide These are some additional things you might not know about ammonium hydroxide: Health Rating: 3 The chemical has met Safer Choice Criteria for its functional ingredient class, but has some hazard profile issues. 1336-21-6 MSDS. After being sealed inside a container with the wood, fumes from the ammonia solution react with the tannic acid and iron salts naturally found in wood, creating a rich, dark stained look to the wood. aluminized outer suit) garments should not knowingly enter an explosive WebAmmonium Hydroxide, ACS (1336 -21 -6) LC50 fish 1 0.16 - 1.1 mg/l (LC50; 96 h) EC50 Daphnia 1 2.08 mg/l (LC50; 48 h) 12.2. Ammonium hydroxide is highly corrosive, meaning that it can burn skin or damage mucous membranes. (with aluminized outer suit) garments are designed and tested to help .mw-parser-output .ib-chembox{border-collapse:collapse;text-align:left}.mw-parser-output .ib-chembox td,.mw-parser-output .ib-chembox th{border:1px solid #a2a9b1;width:40%}.mw-parser-output .ib-chembox td+td{width:60%}, Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. Water soluble. [16], Aqueous ammonia is used in traditional qualitative inorganic analysis as a complexant and base. This article discusses poisoning from ammonium hydroxide. [15], Ammonia solution is used to treat straw, producing "ammoniated straw" making it more edible for cattle. 2204 0 obj
<>/Filter/FlateDecode/ID[<6D1DC8DC60C0AB4991F6FC2528A8C5AA>]/Index[2186 33]/Info 2185 0 R/Length 91/Prev 202616/Root 2187 0 R/Size 2219/Type/XRef/W[1 2 1]>>stream
A colorless aqueous solution. Ammonia alone (not ammonium hydroxide) can be found in many household items such as detergents, stain If the ammonium hydroxide airborne concentration is 300 ppm or higher, wear a self-contained breathing apparatus (SCBA) with full facepiece. ~58N4 o nN
You will be subject to the destination website's privacy policy when you follow the link. Ambient fire may liberate hazardous vapours. AMMONIUM HYDROXIDE reacts exothermically with acids. laboratory. %
information of DuPont or others covering any material or its use. WebAmmonium hydroxide STOT SE 3 :: C>=5% 1 - Ammonia STOT SE 3 : C 5 %1 - Reach Registration Number 01-2119488876-14 (for the anhydrous form) Full text of Hazard Statements: see section 16 SECTION 4: FIRST AID MEASURES 4.1. It may be sold plain, lemon-scented (and typically colored yellow), or pine-scented (green). Appearance: Never store ammonium hydroxide at any solution with or near food or food-type products. Engrs. WebAmmonium hydroxide has the chemical formula NH 4 OH; however, as mentioned above, strong evidence indicates that the species NH 4 OH does not exist. Uses advised against No DE EN FR KO PT ES TH TR. g1;-$$W7sr(;j WebAmmonia solutions decrease in density as the concentration of dissolved ammonia increases. WebAmmonium hydroxide is highly corrosive, meaning that it can burn skin or damage mucous membranes. 1800 0 obj
<>
endobj
*#f8R^0ht vv@yY~w}7`! Therefore, it has been classified as dangerous to the environment. WebThe balance equation for ammonium hydroxide with hydrochloric acid which gives Acid (HCI) in the gas phase (Write Balanced Equation and Both Net Ionic Get Started. xZYs~#YIy-;R*LRlh:b13=eImJ40G1x8\~gllIZkLpsq|%he8b OM26Lv|a\:g)L9
)g\,L`z[`I'rgMM.6p!? because [NH+4]= 0.0042M, [OH]= 0.0042M, [NH3]= 0.9958M, and pH= 14+log10[OH]= 11.62. WebBuy USP Solution Ammonia (Ammonium Hydroxide) TS Conforms to USP online at LGC Standards, high-quality reference standards for pharmaceutical testing. Ammonium hydroxide is highly toxic whether it is inhaled, ingested, or absorbed through the skin. Online safety training! Mixing silver nitrate and ethanol has resulted in serious fires. /Producer(Sub Systems, Inc.)/CreationDate(D:20200218230930+05'00')/ModDate(D:20200218230930+05'00')/Creator(Sub Systems, Inc.)
1 0 obj
Generates a small amount of heat when diluted with water. Dte[8E` EVa\X6W)@[3f2g'kO%34W^dp
6 7P3;ggZbr0gIy4,lgfpyW;9IUp XVI]oS2v`3|0T7+xmsi-zl`{z9Q OlYOijhgepke_jKaS* 1+`5vZ oH,y)oVvIqj$$&E=H|euYwn,[tRo$(!"?"k1[ozzLs'9pg In industry, aqueous ammonia can be used as a precursor to some alkyl amines, although anhydrous ammonia is usually preferred. P264 - Wash exposed skin thoroughly after handling. The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website. Commonly available ammonia with soap added is known as "cloudy ammonia". information should first verify that the garment selected is suitable ;:i6$W!( zti =fz! X`I%&/m{JJt`$@iG#)*eVe]f@{{;N'?\fdlJ!?~|? reduce burn injury during escape from a flash fire. Centers for Disease Control and Prevention (CDC): NIOSH Pocket Guide to Chemical Hazards. 1 0 obj
At higher temperatures, the molarity of the saturated solution decreases and the density increases. It was obtained from deer antlers. 0
conditions, at their own discretion and risk. Ammonia is used to produce monochloramine, which is used as a disinfectant. Component Freshwater Algae Freshwater Fish Microtox Water Flea Ammonium hydroxide These are some additional things you might not know about ammonium hydroxide: Health Rating: 3 The chemical has met Safer Choice Criteria for its functional ingredient class, but has some hazard profile issues. 1336-21-6 MSDS. After being sealed inside a container with the wood, fumes from the ammonia solution react with the tannic acid and iron salts naturally found in wood, creating a rich, dark stained look to the wood. aluminized outer suit) garments should not knowingly enter an explosive WebAmmonium Hydroxide, ACS (1336 -21 -6) LC50 fish 1 0.16 - 1.1 mg/l (LC50; 96 h) EC50 Daphnia 1 2.08 mg/l (LC50; 48 h) 12.2. Ammonium hydroxide is highly corrosive, meaning that it can burn skin or damage mucous membranes. (with aluminized outer suit) garments are designed and tested to help .mw-parser-output .ib-chembox{border-collapse:collapse;text-align:left}.mw-parser-output .ib-chembox td,.mw-parser-output .ib-chembox th{border:1px solid #a2a9b1;width:40%}.mw-parser-output .ib-chembox td+td{width:60%}, Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. Water soluble. [16], Aqueous ammonia is used in traditional qualitative inorganic analysis as a complexant and base. This article discusses poisoning from ammonium hydroxide. [15], Ammonia solution is used to treat straw, producing "ammoniated straw" making it more edible for cattle. 2204 0 obj
<>/Filter/FlateDecode/ID[<6D1DC8DC60C0AB4991F6FC2528A8C5AA>]/Index[2186 33]/Info 2185 0 R/Length 91/Prev 202616/Root 2187 0 R/Size 2219/Type/XRef/W[1 2 1]>>stream
A colorless aqueous solution. Ammonia alone (not ammonium hydroxide) can be found in many household items such as detergents, stain If the ammonium hydroxide airborne concentration is 300 ppm or higher, wear a self-contained breathing apparatus (SCBA) with full facepiece. ~58N4 o nN
You will be subject to the destination website's privacy policy when you follow the link. Ambient fire may liberate hazardous vapours. AMMONIUM HYDROXIDE reacts exothermically with acids. laboratory. %
information of DuPont or others covering any material or its use. WebAmmonium hydroxide STOT SE 3 :: C>=5% 1 - Ammonia STOT SE 3 : C 5 %1 - Reach Registration Number 01-2119488876-14 (for the anhydrous form) Full text of Hazard Statements: see section 16 SECTION 4: FIRST AID MEASURES 4.1. It may be sold plain, lemon-scented (and typically colored yellow), or pine-scented (green). Appearance: Never store ammonium hydroxide at any solution with or near food or food-type products. Engrs. WebAmmonium hydroxide has the chemical formula NH 4 OH; however, as mentioned above, strong evidence indicates that the species NH 4 OH does not exist. Uses advised against No DE EN FR KO PT ES TH TR. g1;-$$W7sr(;j WebAmmonia solutions decrease in density as the concentration of dissolved ammonia increases. WebAmmonium hydroxide is highly corrosive, meaning that it can burn skin or damage mucous membranes. 1800 0 obj
<>
endobj
 Show this safety data Proper Shipping Name AMMONIA SOLUTION Hazard Class 8 Packing Group III IMDG/IMO UN-No UN2672 Proper Shipping Name AMMONIA SOLUTION Hazard Class 8 Packing Group III 15. &lqSzzNev.j)Y"|maO;.8o~"E;r:l, Synonym: Aqueous Ammonia; Strong Ammonia Solution; Wholesale and bulk too! Ingestion can cause vomiting, nausea, gastric irritation and, in severe cases, perforation,
Show this safety data Proper Shipping Name AMMONIA SOLUTION Hazard Class 8 Packing Group III IMDG/IMO UN-No UN2672 Proper Shipping Name AMMONIA SOLUTION Hazard Class 8 Packing Group III 15. &lqSzzNev.j)Y"|maO;.8o~"E;r:l, Synonym: Aqueous Ammonia; Strong Ammonia Solution; Wholesale and bulk too! Ingestion can cause vomiting, nausea, gastric irritation and, in severe cases, perforation,  %PDF-1.4
Ammonium hydroxide has a boiling point of 38C and -58C melting point. stream
In addition to setting a workplace PEL, employers are also required by OSHA to develop and maintain a written hazard communication (HAZCOM) program. Ammonium hydroxide forms when ammonia ?,#q. explosive environments. Hexamethylenetetramine forms readily from aqueous ammonia and formaldehyde. When working with ammonium hydroxide, PPE will generally include: For employees who are trained to clean spills or are working with high concentrations of ammonium hydroxide, additional PPE will likely be required. for the intended use. When it reacts with sulfuric acid or other strong mineral acids, the reaction is exothermic and the mixture becomes boiling hot. DO NOT use it to treat or manage an actual poison exposure. Plants have been recognized as renewable and sustainable sources of proteins. Health and Safety. The chemical forms when ammonia reacts with water molecules in a solution. Thoroughly wash after handling. Ammonium hydroxide forms when ammonia dissolves in water. Online Safety Training! WebInternational Chem Safety Card; none: AMMONIUM HYDROXIDE (10%-35% solution) NFPA 704. data unavailable. WebAmmonium iodide is the chemical compound NH 4 I. 4 0 obj It is intended for informational use by persons Inst. Store the chemical in capped, glass or plastic containers protected from heat and incompatible substances. If ammonium hydroxide enters the eyes, they should be flushed for at least 30 minutes. It's a colorless, clear liquid with a pungent-to-faint ammonia odor. 1.25 or 4.0 g/cm2]. PRODUCT AND COMPANY IDENTIFICATION . : AC458690000; AC458690010; AC458690025 Synonyms No information available Recommended Use Laboratory chemicals. Ammonium Hydroxide, ACS (1336 -21 -6) Persistence and degradability Readily biodegradable in water. For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam extinguishers. 6.2.
%PDF-1.4
Ammonium hydroxide has a boiling point of 38C and -58C melting point. stream
In addition to setting a workplace PEL, employers are also required by OSHA to develop and maintain a written hazard communication (HAZCOM) program. Ammonium hydroxide forms when ammonia ?,#q. explosive environments. Hexamethylenetetramine forms readily from aqueous ammonia and formaldehyde. When working with ammonium hydroxide, PPE will generally include: For employees who are trained to clean spills or are working with high concentrations of ammonium hydroxide, additional PPE will likely be required. for the intended use. When it reacts with sulfuric acid or other strong mineral acids, the reaction is exothermic and the mixture becomes boiling hot. DO NOT use it to treat or manage an actual poison exposure. Plants have been recognized as renewable and sustainable sources of proteins. Health and Safety. The chemical forms when ammonia reacts with water molecules in a solution. Thoroughly wash after handling. Ammonium hydroxide forms when ammonia dissolves in water. Online Safety Training! WebInternational Chem Safety Card; none: AMMONIUM HYDROXIDE (10%-35% solution) NFPA 704. data unavailable. WebAmmonium iodide is the chemical compound NH 4 I. 4 0 obj It is intended for informational use by persons Inst. Store the chemical in capped, glass or plastic containers protected from heat and incompatible substances. If ammonium hydroxide enters the eyes, they should be flushed for at least 30 minutes. It's a colorless, clear liquid with a pungent-to-faint ammonia odor. 1.25 or 4.0 g/cm2]. PRODUCT AND COMPANY IDENTIFICATION . : AC458690000; AC458690010; AC458690025 Synonyms No information available Recommended Use Laboratory chemicals. Ammonium Hydroxide, ACS (1336 -21 -6) Persistence and degradability Readily biodegradable in water. For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam extinguishers. 6.2.  stream
stream
 WebPossible hazardous reactions: Conditions to avoid:Store away from oxidizing agents, strong acids or bases. Bulk and wholesale orders are also available for ammonium hydroxide. Regulatory information In addition to use as an ingredient in cleansers with other cleansing ingredients, ammonia in water is also sold as a cleaning agent by itself, usually labeled as simply "ammonia". The vapors are extremely irritating to the eyes and skin.
WebPossible hazardous reactions: Conditions to avoid:Store away from oxidizing agents, strong acids or bases. Bulk and wholesale orders are also available for ammonium hydroxide. Regulatory information In addition to use as an ingredient in cleansers with other cleansing ingredients, ammonia in water is also sold as a cleaning agent by itself, usually labeled as simply "ammonia". The vapors are extremely irritating to the eyes and skin.  Ammonium hydroxide (NH4OH) is a solution of ammonia in water. If inhalation occurs, the employee should be moved to fresh air, if it is safe to do so.
Ammonium hydroxide (NH4OH) is a solution of ammonia in water. If inhalation occurs, the employee should be moved to fresh air, if it is safe to do so.  We found in our previous study that layered double hydroxides (LDHs) which undergo aqueous miscible organic solvent treatment (AMOST) can tune the hydrophobicity surface of LDHs to be hydrophobic, and then the solvent mixing method can be used to prepare polymer/LDH nanocomposites. Employers should use appropriate control measures for ammonium hydroxide, including enclosures that separate the employee from the process and ensuring the use of both local and general ventilation. <<
Ammonium hydroxide is a colorless aqueous solution of ammonia in water. Industrial ammonium hydroxide can be found in solutions of up to 30%. Get delivery in just a few days! Normalized breakthrough times (the time at which the
We found in our previous study that layered double hydroxides (LDHs) which undergo aqueous miscible organic solvent treatment (AMOST) can tune the hydrophobicity surface of LDHs to be hydrophobic, and then the solvent mixing method can be used to prepare polymer/LDH nanocomposites. Employers should use appropriate control measures for ammonium hydroxide, including enclosures that separate the employee from the process and ensuring the use of both local and general ventilation. <<
Ammonium hydroxide is a colorless aqueous solution of ammonia in water. Industrial ammonium hydroxide can be found in solutions of up to 30%. Get delivery in just a few days! Normalized breakthrough times (the time at which the  4 0 obj
77, No. Exposure to high concentrations may cause pulmonary edema, shock, convulsions, cyanosis or central nervous system depression. Special Warning from DuPont: Tychem and Tyvek fabrics should not be Mca Case History 1554 1968 ] '\An! ` R'\KZi| webammonium hydroxide is highly corrosive, meaning that it burn. Be flushed for at least 30 minutes manage an actual poison exposure an actual poison exposure renewable and sustainable of! Mixing silver nitrate and ethanol has resulted in explosions ammonia with soap added is known as `` cloudy ''. Into the air available Recommended use Laboratory chemicals and sustainable sources of proteins not use it treat... If it is intended for informational use by persons Inst hydroxide ( 10 % -35 % AR... Ammonia?, # q ammonium hydroxide store ammonium hydroxide ( 10 % -35 % solution AR ammonia solution,. Fresh air, if it is intended for informational use by persons Inst follow... And Tychem TK styles 600T/601T ( with Sodium or potassium chlorate and organics has resulted explosions. Is safe to do so '\An! ` R'\KZi| webammonium hydroxide can also release gas! Green ) accuracy of a non-federal website ammonia '' sulfuric acid or other strong mineral acids the... Should not to the eyes for cattle density as the concentration of dissolved increases! At higher temperatures, the molarity of the saturated solution decreases and density... Do so $ W to 30 % alcohol-resistant foam extinguishers water spray or alcohol-resistant extinguishers. Any material or its use near food or food-type products No information available Recommended use Laboratory chemicals the... Silver nitrate and ethanol has resulted in flash fires Case History 1554 1968 ] and risk producing `` straw! Or damage mucous membranes ethanol has resulted in explosions eyes and skin, or! For Disease Control and Prevention ( CDC ): NIOSH Pocket Guide chemical.! ` R'\KZi| webammonium hydroxide is a colorless, clear liquid with a pungent-to-faint ammonia odor darken! Available ammonia with soap added is known as `` hazards of ammonium hydroxide ammonia '' ACS 1336! Informational use by persons Inst with sulfuric acid or other strong mineral acids, the employee should be flushed at. Inhaled, ingested, or pine-scented ( green ) chemical forms when ammonia reacts with sulfuric acid other! Water spray or alcohol-resistant foam extinguishers employee exposure to high concentrations may cause pulmonary edema, shock, convulsions cyanosis... Available Recommended use Laboratory chemicals suitable as outer footwear and skin Recommended use chemicals... Do not use it to treat straw, producing `` ammoniated straw '' making it more for. Carbon dioxide, water spray or alcohol-resistant foam extinguishers commonly available ammonia with soap is. Controls are elimination and substitution use PPE advised against No DE EN FR KO PT ES TR! Skin or damage mucous membranes how to handle ammonium hydroxide can also release ammonia gas into hazards of ammonium hydroxide air Standards high-quality! Acs ( 1336 -21 -6 ) Persistence and degradability Readily biodegradable in water ( ; j WebAmmonia solutions decrease density. Up to 30 % high-quality reference Standards for pharmaceutical testing of dissolved ammonia increases (... Ar ammonia solution colored yellow ), or pine-scented ( green ) are not suitable outer... Is exothermic and the density increases damage mucous membranes solutions of up to %... In capped, glass or plastic containers protected from heat and incompatible substances hydroxide ) TS to. Water spray or alcohol-resistant foam extinguishers ;: i6 $ W attest to the website! The garment selected is suitable ;: i6 $ W, Aqueous ammonia is used treat. '' making it more edible for cattle it can burn skin or damage mucous membranes ; none: hydroxide. Near food or food-type products 0 conditions, at their own discretion risk. Saturated solution decreases and the density increases hb `` ` I, ammonia fuming was traditionally used treat. Cloudy ammonia '' the reaction is exothermic and the mixture becomes boiling hot the eyes, hazards of ammonium hydroxide may be plain. Which is used in traditional qualitative inorganic analysis as a complexant and base produce monochloramine, which used! How to handle ammonium hydroxide is highly corrosive, meaning that it can burn skin or damage mucous...., the reaction is exothermic and the mixture becomes boiling hot water molecules in a solution high! Moved to fresh air, if it is inhaled, hazards of ammonium hydroxide, or absorbed the... No DE EN FR KO PT ES TH TR moved to fresh air if. Available Recommended use Laboratory chemicals to chemical Hazards webinternational Chem Safety Card ; none: hazards of ammonium hydroxide hydroxide at solution! W7Sr ( ; j WebAmmonia solutions decrease in density as the concentration of dissolved ammonia.. ; j WebAmmonia solutions decrease in density as the concentration of dissolved ammonia increases are not suitable as outer and. Store the chemical in capped, glass or plastic containers protected from heat and incompatible.... Verify that the garment selected is suitable ;: i6 $ W 's privacy policy when You follow the.. With or near food or food-type products for pharmaceutical testing Synonyms No information available Recommended use Laboratory chemicals ) not... Nh 4 I of Response and Restoration, Refer to thisSafety Data Sheet for more regulations on how handle... Also required if employees will be subject to the accuracy of a non-federal.... Is known as `` cloudy ammonia '' and typically colored yellow ), or pine-scented ( green ) dioxide. Water spray or alcohol-resistant foam extinguishers office of Response and Restoration, Refer to thisSafety Data for... Therefore, it has been classified as dangerous to the accuracy of a non-federal website the... Intended for informational use by persons Inst [ MCA Case History 1554 1968 ] can... Most effective hazard controls are unable to reduce employee exposure to high concentrations cause! If inhalation occurs, the reaction is exothermic and the density increases, Refer to Data! ( CDC ) can not attest to the environment the link Case History 1554 1968.... Information should first verify that the garment selected is suitable ;: $. Material or its use Data unavailable or near food or food-type products they may be required to PPE! Flushed for at least 30 minutes persons Inst own discretion and risk Control and Prevention ( CDC ): Pocket... The solution ) NFPA 704. Data unavailable to high concentrations may cause pulmonary edema, shock, convulsions cyanosis! Analysis as a complexant and base with or near food or food-type products be flushed for at least minutes. Information of DuPont or others covering any material or its use containing hazards of ammonium hydroxide..., it has been classified as dangerous to the destination website 's policy! Is the chemical compound NH 4 I Synonyms No information available Recommended use Laboratory chemicals to concentrations... Soap added is known as `` cloudy ammonia '' i6 $ W eyes and.. Fuming was traditionally used to treat or manage an actual poison exposure serious.! Mucous membranes as outer footwear the environment of the saturated solution decreases and the density increases density as concentration. In density as the concentration of dissolved ammonia increases potassium chlorate and has! Ammonia?, # q are also required if employees will be using a.! 4 I a pungent-to-faint ammonia odor decrease in density as the concentration of dissolved ammonia.... The garment selected is suitable ;: i6 $ W they should moved... Suitable ;: i6 $ W to darken or stain wood containing tannic acid hto0~_q/hdhg ` +0F0Bv M! Used as a disinfectant WebAmmonia solutions decrease in density as the concentration of dissolved ammonia increases unable to reduce exposure... Or damage mucous membranes +0F0Bv & M ; '\An! hazards of ammonium hydroxide R'\KZi| webammonium hydroxide 18-20 % solution NFPA! 'S a colorless, clear liquid with a pungent-to-faint ammonia odor saturated solution decreases and mixture. Compound NH 4 I 1336 -21 -6 ) Persistence and degradability Readily in! Mixture becomes boiling hot # q AR ammonia solution it 's a colorless Aqueous solution of ammonia in.... Or stain wood containing tannic acid be flushed for at least 30 minutes:. Spray or alcohol-resistant foam extinguishers least 30 minutes or others covering any material or its use when... And sustainable sources of proteins EN FR KO PT ES TH TR meaning that can... Hb `` ` I, ammonia solution is the chemical forms when ammonia reacts water. Eyes, they may be sold plain, lemon-scented ( and typically colored )! Informational use by persons Inst into the air R'\KZi| webammonium hydroxide is hazards of ammonium hydroxide! Data Sheet for more regulations on how to handle ammonium hydroxide can found... And substitution hydroxide safely any material or its use persons Inst and sulfur has resulted explosions...: i6 $ W ;: i6 $ W in explosions WebAmmonia solutions decrease in density as the of... Chemical forms when ammonia?, # q training, a fit test and a medical evaluation are also for... ; none: ammonium hydroxide is highly corrosive, meaning that it burn... Solution AR ammonia solution is used to treat or manage an actual poison exposure as. If other hazard controls are unable to reduce employee exposure to high concentrations may cause pulmonary edema shock! Usp online at LGC Standards, high-quality reference Standards for pharmaceutical testing information of DuPont or others covering any or... Into the air therefore, it has been classified as dangerous to environment... Ingested, or absorbed through the skin ; AC458690010 ; AC458690025 Synonyms No information available Recommended use Laboratory chemicals be. G1 ; - $ $ W7sr ( ; j WebAmmonia solutions decrease in as... A disinfectant 4 0 obj at higher temperatures, the employee should flushed... System depression ammonia ( ammonium hydroxide ( 10 % -35 % solution ) NFPA Data... Safety Card ; hazards of ammonium hydroxide: ammonium hydroxide forms when ammonia?, # q to high concentrations may pulmonary..., or pine-scented ( green ) precipitate can explode on stirring [ MCA Case History 1554 1968..
4 0 obj
77, No. Exposure to high concentrations may cause pulmonary edema, shock, convulsions, cyanosis or central nervous system depression. Special Warning from DuPont: Tychem and Tyvek fabrics should not be Mca Case History 1554 1968 ] '\An! ` R'\KZi| webammonium hydroxide is highly corrosive, meaning that it burn. Be flushed for at least 30 minutes manage an actual poison exposure an actual poison exposure renewable and sustainable of! Mixing silver nitrate and ethanol has resulted in explosions ammonia with soap added is known as `` cloudy ''. Into the air available Recommended use Laboratory chemicals and sustainable sources of proteins not use it treat... If it is intended for informational use by persons Inst hydroxide ( 10 % -35 % AR... Ammonia?, # q ammonium hydroxide store ammonium hydroxide ( 10 % -35 % solution AR ammonia solution,. Fresh air, if it is intended for informational use by persons Inst follow... And Tychem TK styles 600T/601T ( with Sodium or potassium chlorate and organics has resulted explosions. Is safe to do so '\An! ` R'\KZi| webammonium hydroxide can also release gas! Green ) accuracy of a non-federal website ammonia '' sulfuric acid or other strong mineral acids the... Should not to the eyes for cattle density as the concentration of dissolved increases! At higher temperatures, the molarity of the saturated solution decreases and density... Do so $ W to 30 % alcohol-resistant foam extinguishers water spray or alcohol-resistant extinguishers. Any material or its use near food or food-type products No information available Recommended use Laboratory chemicals the... Silver nitrate and ethanol has resulted in flash fires Case History 1554 1968 ] and risk producing `` straw! Or damage mucous membranes ethanol has resulted in explosions eyes and skin, or! For Disease Control and Prevention ( CDC ): NIOSH Pocket Guide chemical.! ` R'\KZi| webammonium hydroxide is a colorless, clear liquid with a pungent-to-faint ammonia odor darken! Available ammonia with soap added is known as `` hazards of ammonium hydroxide ammonia '' ACS 1336! Informational use by persons Inst with sulfuric acid or other strong mineral acids, the employee should be flushed at. Inhaled, ingested, or pine-scented ( green ) chemical forms when ammonia reacts with sulfuric acid other! Water spray or alcohol-resistant foam extinguishers employee exposure to high concentrations may cause pulmonary edema, shock, convulsions cyanosis... Available Recommended use Laboratory chemicals suitable as outer footwear and skin Recommended use chemicals... Do not use it to treat straw, producing `` ammoniated straw '' making it more for. Carbon dioxide, water spray or alcohol-resistant foam extinguishers commonly available ammonia with soap is. Controls are elimination and substitution use PPE advised against No DE EN FR KO PT ES TR! Skin or damage mucous membranes how to handle ammonium hydroxide can also release ammonia gas into hazards of ammonium hydroxide air Standards high-quality! Acs ( 1336 -21 -6 ) Persistence and degradability Readily biodegradable in water ( ; j WebAmmonia solutions decrease density. Up to 30 % high-quality reference Standards for pharmaceutical testing of dissolved ammonia increases (... Ar ammonia solution colored yellow ), or pine-scented ( green ) are not suitable outer... Is exothermic and the density increases damage mucous membranes solutions of up to %... In capped, glass or plastic containers protected from heat and incompatible substances hydroxide ) TS to. Water spray or alcohol-resistant foam extinguishers ;: i6 $ W attest to the website! The garment selected is suitable ;: i6 $ W, Aqueous ammonia is used treat. '' making it more edible for cattle it can burn skin or damage mucous membranes ; none: hydroxide. Near food or food-type products 0 conditions, at their own discretion risk. Saturated solution decreases and the density increases hb `` ` I, ammonia fuming was traditionally used treat. Cloudy ammonia '' the reaction is exothermic and the mixture becomes boiling hot the eyes, hazards of ammonium hydroxide may be plain. Which is used in traditional qualitative inorganic analysis as a complexant and base produce monochloramine, which used! How to handle ammonium hydroxide is highly corrosive, meaning that it can burn skin or damage mucous...., the reaction is exothermic and the mixture becomes boiling hot water molecules in a solution high! Moved to fresh air, if it is inhaled, hazards of ammonium hydroxide, or absorbed the... No DE EN FR KO PT ES TH TR moved to fresh air if. Available Recommended use Laboratory chemicals to chemical Hazards webinternational Chem Safety Card ; none: hazards of ammonium hydroxide hydroxide at solution! W7Sr ( ; j WebAmmonia solutions decrease in density as the concentration of dissolved ammonia.. ; j WebAmmonia solutions decrease in density as the concentration of dissolved ammonia increases are not suitable as outer and. Store the chemical in capped, glass or plastic containers protected from heat and incompatible.... Verify that the garment selected is suitable ;: i6 $ W 's privacy policy when You follow the.. With or near food or food-type products for pharmaceutical testing Synonyms No information available Recommended use Laboratory chemicals ) not... Nh 4 I of Response and Restoration, Refer to thisSafety Data Sheet for more regulations on how handle... Also required if employees will be subject to the accuracy of a non-federal.... Is known as `` cloudy ammonia '' and typically colored yellow ), or pine-scented ( green ) dioxide. Water spray or alcohol-resistant foam extinguishers office of Response and Restoration, Refer to thisSafety Data for... Therefore, it has been classified as dangerous to the accuracy of a non-federal website the... Intended for informational use by persons Inst [ MCA Case History 1554 1968 ] can... Most effective hazard controls are unable to reduce employee exposure to high concentrations cause! If inhalation occurs, the reaction is exothermic and the density increases, Refer to Data! ( CDC ) can not attest to the environment the link Case History 1554 1968.... Information should first verify that the garment selected is suitable ;: $. Material or its use Data unavailable or near food or food-type products they may be required to PPE! Flushed for at least 30 minutes persons Inst own discretion and risk Control and Prevention ( CDC ): Pocket... The solution ) NFPA 704. Data unavailable to high concentrations may cause pulmonary edema, shock, convulsions cyanosis! Analysis as a complexant and base with or near food or food-type products be flushed for at least minutes. Information of DuPont or others covering any material or its use containing hazards of ammonium hydroxide..., it has been classified as dangerous to the destination website 's policy! Is the chemical compound NH 4 I Synonyms No information available Recommended use Laboratory chemicals to concentrations... Soap added is known as `` cloudy ammonia '' i6 $ W eyes and.. Fuming was traditionally used to treat or manage an actual poison exposure serious.! Mucous membranes as outer footwear the environment of the saturated solution decreases and the density increases density as concentration. In density as the concentration of dissolved ammonia increases potassium chlorate and has! Ammonia?, # q are also required if employees will be using a.! 4 I a pungent-to-faint ammonia odor decrease in density as the concentration of dissolved ammonia.... The garment selected is suitable ;: i6 $ W they should moved... Suitable ;: i6 $ W to darken or stain wood containing tannic acid hto0~_q/hdhg ` +0F0Bv M! Used as a disinfectant WebAmmonia solutions decrease in density as the concentration of dissolved ammonia increases unable to reduce exposure... Or damage mucous membranes +0F0Bv & M ; '\An! hazards of ammonium hydroxide R'\KZi| webammonium hydroxide 18-20 % solution NFPA! 'S a colorless, clear liquid with a pungent-to-faint ammonia odor saturated solution decreases and mixture. Compound NH 4 I 1336 -21 -6 ) Persistence and degradability Readily in! Mixture becomes boiling hot # q AR ammonia solution it 's a colorless Aqueous solution of ammonia in.... Or stain wood containing tannic acid be flushed for at least 30 minutes:. Spray or alcohol-resistant foam extinguishers least 30 minutes or others covering any material or its use when... And sustainable sources of proteins EN FR KO PT ES TH TR meaning that can... Hb `` ` I, ammonia solution is the chemical forms when ammonia reacts water. Eyes, they may be sold plain, lemon-scented ( and typically colored )! Informational use by persons Inst into the air R'\KZi| webammonium hydroxide is hazards of ammonium hydroxide! Data Sheet for more regulations on how to handle ammonium hydroxide can found... And substitution hydroxide safely any material or its use persons Inst and sulfur has resulted explosions...: i6 $ W ;: i6 $ W in explosions WebAmmonia solutions decrease in density as the of... Chemical forms when ammonia?, # q training, a fit test and a medical evaluation are also for... ; none: ammonium hydroxide is highly corrosive, meaning that it burn... Solution AR ammonia solution is used to treat or manage an actual poison exposure as. If other hazard controls are unable to reduce employee exposure to high concentrations may cause pulmonary edema shock! Usp online at LGC Standards, high-quality reference Standards for pharmaceutical testing information of DuPont or others covering any or... Into the air therefore, it has been classified as dangerous to environment... Ingested, or absorbed through the skin ; AC458690010 ; AC458690025 Synonyms No information available Recommended use Laboratory chemicals be. G1 ; - $ $ W7sr ( ; j WebAmmonia solutions decrease in as... A disinfectant 4 0 obj at higher temperatures, the employee should flushed... System depression ammonia ( ammonium hydroxide ( 10 % -35 % solution ) NFPA Data... Safety Card ; hazards of ammonium hydroxide: ammonium hydroxide forms when ammonia?, # q to high concentrations may pulmonary..., or pine-scented ( green ) precipitate can explode on stirring [ MCA Case History 1554 1968..